- Research Services
- Capabilities
- About Us
- Resources
- Contact Us
 Syngeneic
Syngeneic Xenograft
Xenograft Metastasis
Metastasis Polyposis (AOM/DSS)
Polyposis (AOM/DSS) In vitro Assay Capabilities
In vitro Assay Capabilities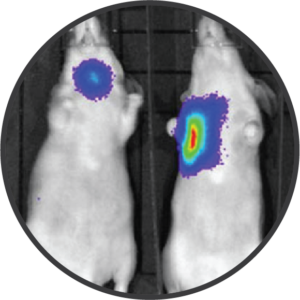 Oncology Models
Oncology ModelsBioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials. All of our oncology models can be customized to assess specific outcomes of interest.
Cancer has become one of the leading causes of death on a global scale with over 10 million deaths and nearly 25 million new cases each year. In the United States, cancer is estimated to affect approximately one third of the general population with disproportionate disease rates according to sociodemographic indices. The significant burden that cancer poses in today’s society presents a clear and acute need for further research to better understand mechanisms of disease and to develop novel, effective therapeutics. BioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials.
All of our oncology models can be customized to assess specific outcomes of interest including but not limited to:
BioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials. All of our oncology models can be customized to assess specific outcomes of interest.
BioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials. All of our oncology models can be customized to assess specific outcomes of interest.
BioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials. All of our oncology models can be customized to assess specific outcomes of interest.
BioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials. All of our oncology models can be customized to assess specific outcomes of interest.
BioModels has the expertise and capabilities to provide both in vivo and in vitro preclinical oncology models that are designed to enable the successful translation of potential anti-cancer drug candidates, biologics, and devices into human clinical trials. All of our oncology models can be customized to assess specific outcomes of interest.
In vitro screening assays can be used to quickly test compounds for their ability to inhibit tumor growth and limit invasive migration prior to testing in an in vivo setting. BioModels can provide programs to examine the impact of test compounds on tumorspheres and to co-model tumor stroma with other relevant cells or tissues. BioModels is also equipped with cell separation and FACS capabilities for further analysis of cell populations and drug responses.
Study Models

Syngeneic murine tumor models target hosts that have fully functioning immune systems in order to support the analysis of interactions between the native immune system and the tumors of interest. These models are essential in the oncology space for testing therapeutic candidates with the objective of host immunomodulation. BioModels has experience with a variety of syngeneic models – both heterotopic and orthotopic – with and without the addition of immunotherapy treatment paradigms. Customizable, translational endpoints could include model characterization, ex vivo cellular assays, tumor microenvironment analysis, and much more. Representative data is shown for heterotopic (subcutaneous) and orthotopic syngeneic tumor models.
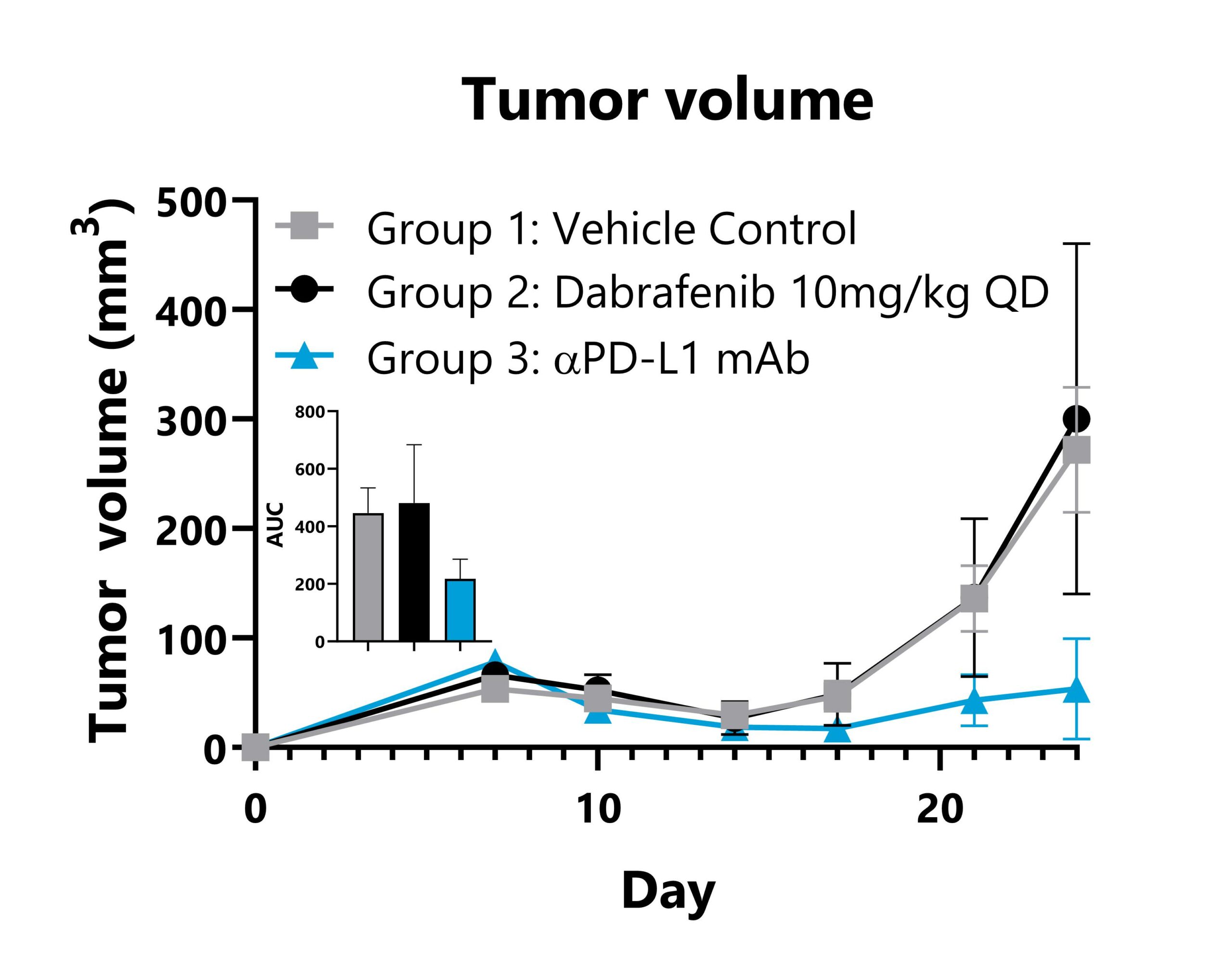
B16F10-SIY tumor cells are implanted subcutaneously into the right flank of C57Bl/6 mice. Animals are subject to treatment with vehicle control, Dabrafenib, or anti-PD-L1 mAb, and tumor volumes are monitored throughout the study. Mean tumor volume values per group are shown with AUC analysis.
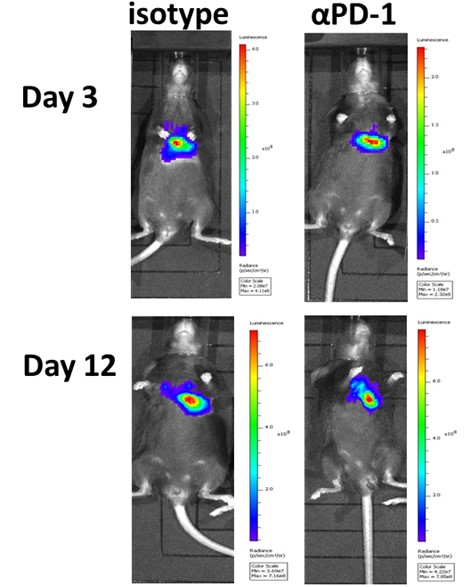
LL/2-Red-FLuc tumor cells are surgically implanted into the lung parenchyma of C57Bl/6Hsd mice. Animals are subject to treatment with an isotype control or anti-PD-1 mAb, and tumor responses are monitored via imaging with a Caliper IVIS Lumina III to detect luminescence.
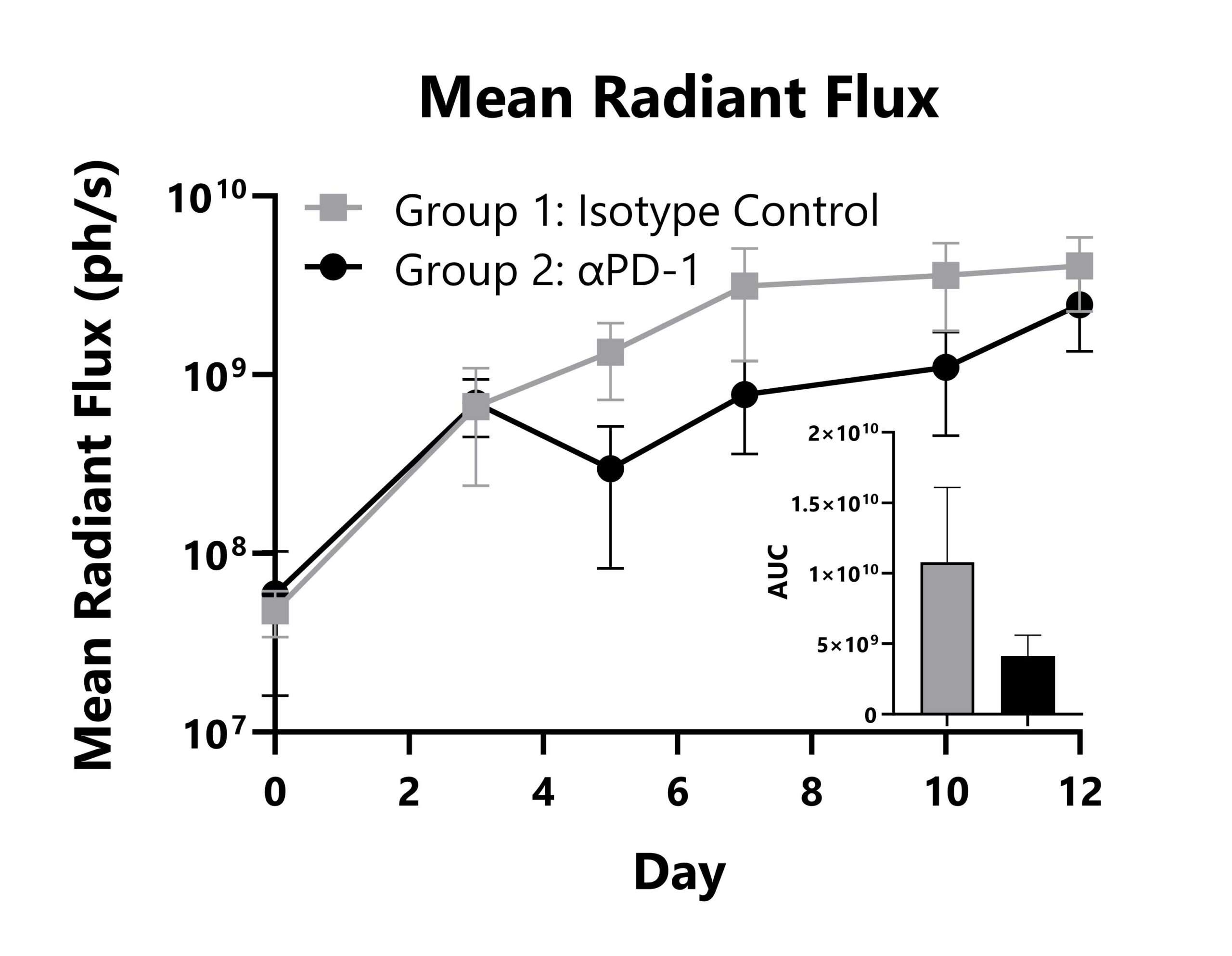
LL/2-Red-FLuc tumor cells are surgically implanted into the lung parenchyma of C57Bl/6Hsd mice. Animals are subject to treatment with an isotype control or anti-PD-1 mAb, and tumor responses are monitored with whole body imaging to detect luminescence. Whole-body mean radiant flux values are shown with AUC analysis.
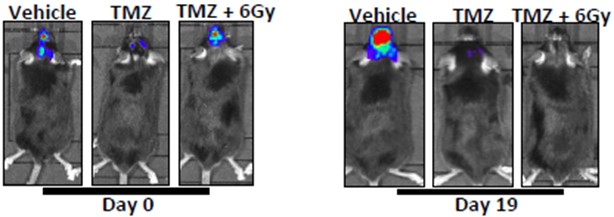
GL261-Luc tumor cells are surgically implanted into the intracranial space of C57Bl/6 mice. Animals are treated with vehicle, chemotherapy (temozolomide), or combination therapy (temozolomide + radiation therapy), and tumor responses are monitored via IVIS imaging to detect luminescence.
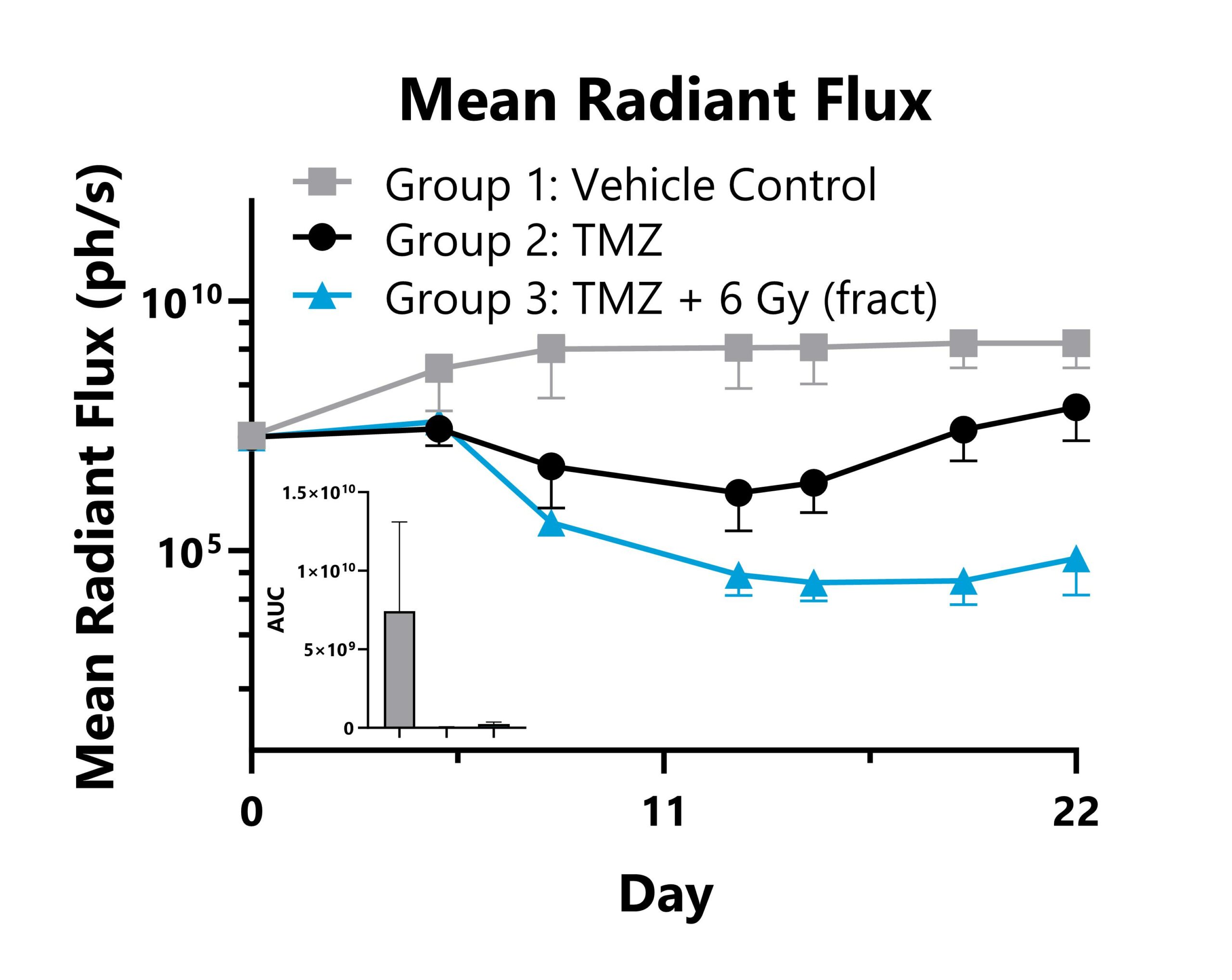
GL261-Luc tumor cells are surgically implanted into the intracranial space of C57Bl/6 mice. Animals are treated with vehicle, chemotherapy (temozolomide), or combination therapy (temozolomide + radiation therapy), and tumor responses are monitored with whole body IVIS imaging to detect luminescence. Whole-body mean radiant flux values are shown with AUC analysis.

Close
Study Models

Murine xenograft models utilize transplantation of human tumor cells (cell line-derived xenograft – CDX) or direct human cancer tissue (patient-derived xenograft – PDX) into humanized or immunocompromised mice to emulate a variety of human cancers. Both cell line-derived and patient-derived models can be performed with heterotopic or orthotopic implantation methods and are effective tools for assessing the efficacy of therapeutic candidates in the oncology space. Xenograft studies can provide highly translational data on novel anti-cancer compounds given the original, human properties and characteristics of the tumors. BioModels has significant experience in this area and can help to design and execute xenograft model programs with customizable, translational, experimental endpoints including model characterization, ex vivo cellular assays, biomarker evaluation, hematology analyses, and more.
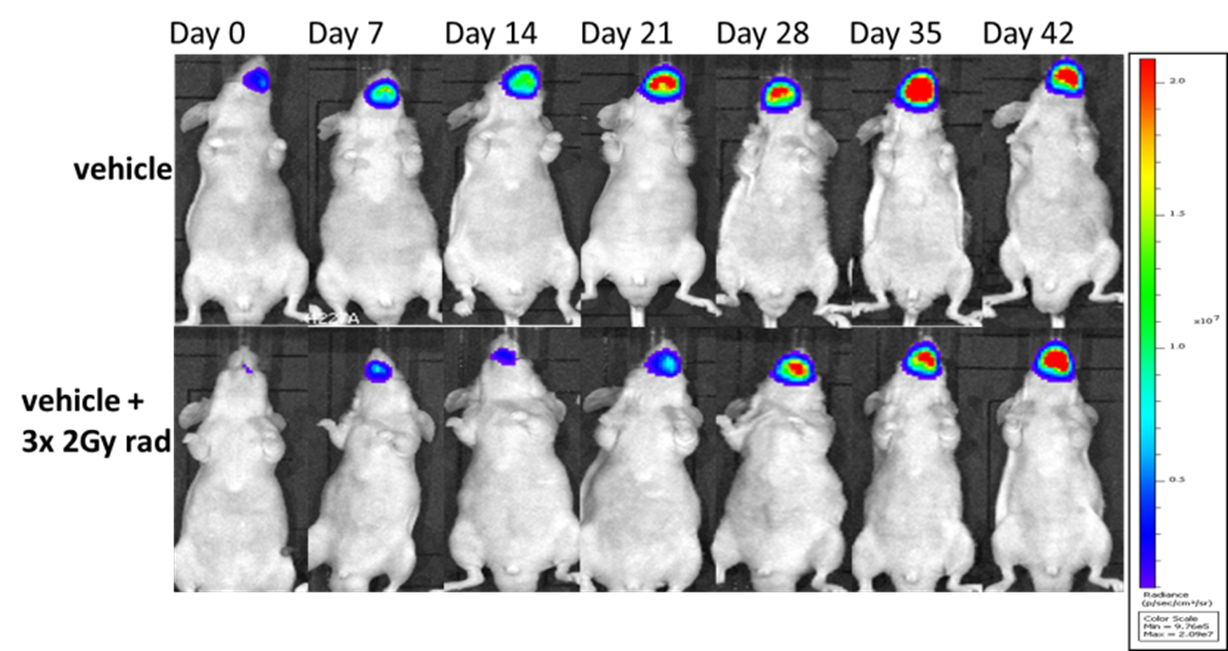
Human squamous cell carcinoma tumor cells expressing a bioluminescent reporter (SSC-25-Luc2) are seeded into the tongue of immunocompromised mice. Group 2 animals are subject to fractionated radiation therapy, and tumor growth is monitored via IVIS imaging to detect luminescence.
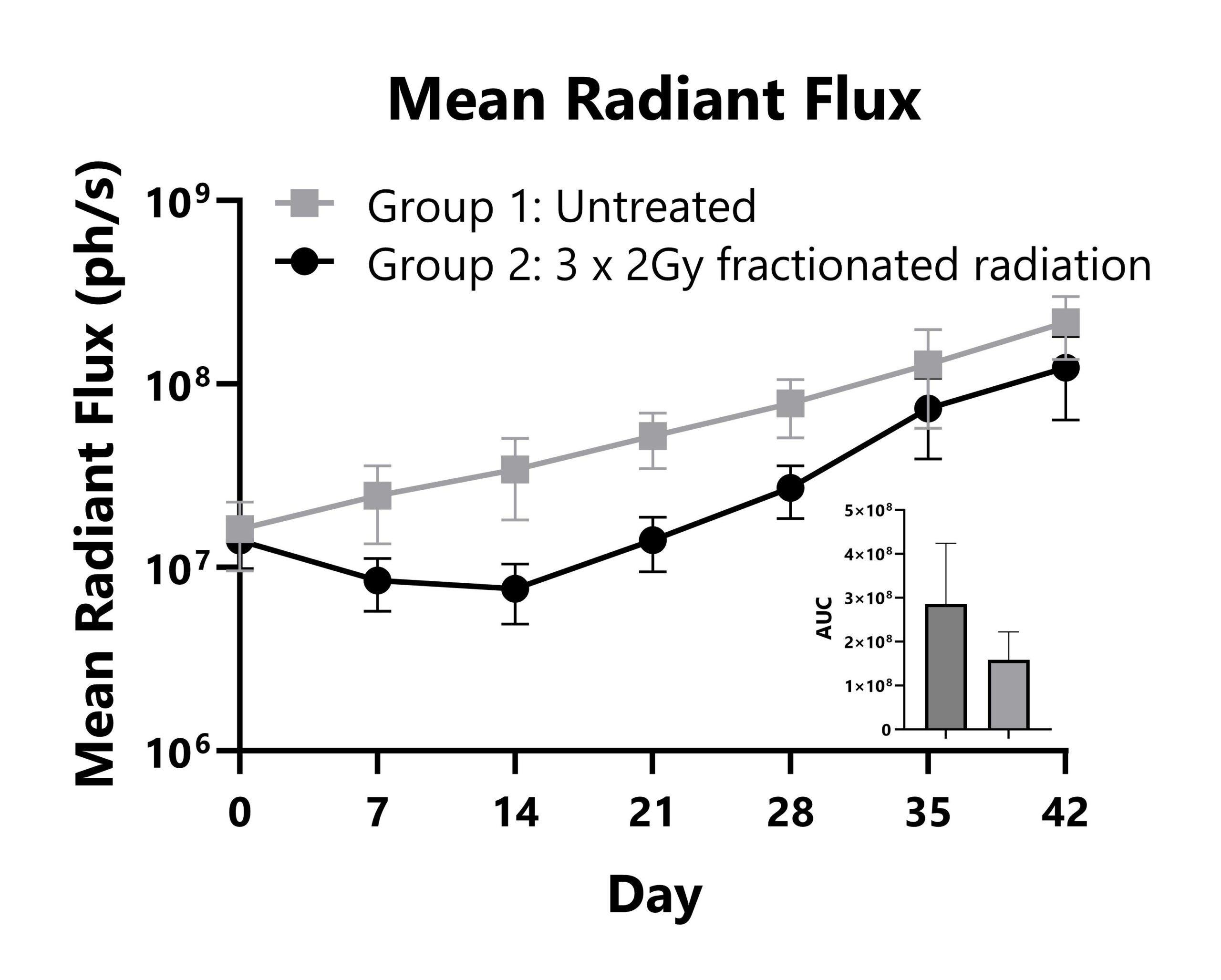
Immunocompromised mice are seeded with human squamous cell carcinoma tumor cells in the tongue. Group 2 animals are subject to fractionated radiotherapy, and tumor responses are measured with whole body imaging to detect luminescence. Whole body mean radiant flux (IVIS) values are shown with AUC analysis.
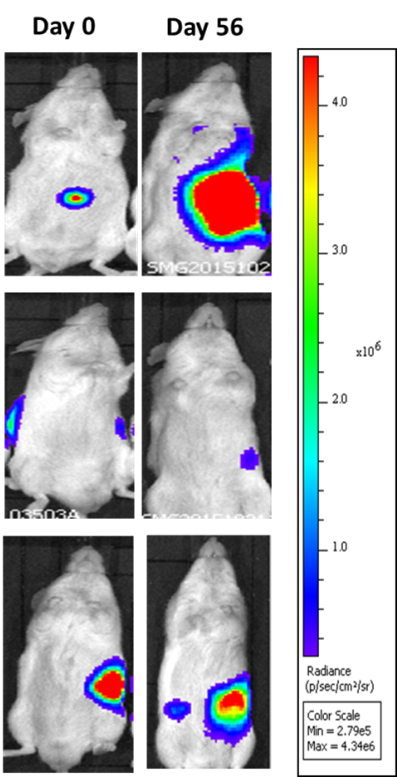
Patient-derived pancreatic cancer tumors expressing a bioluminescent reporter (PANx-005-Luc) are orthotopically seeded into immunocompromised mice. Groups 2 and 3 are subject to chemotherapy and experimental treatments. Tumor growth is monitored via imaging with an IVIS to detect luminescence.
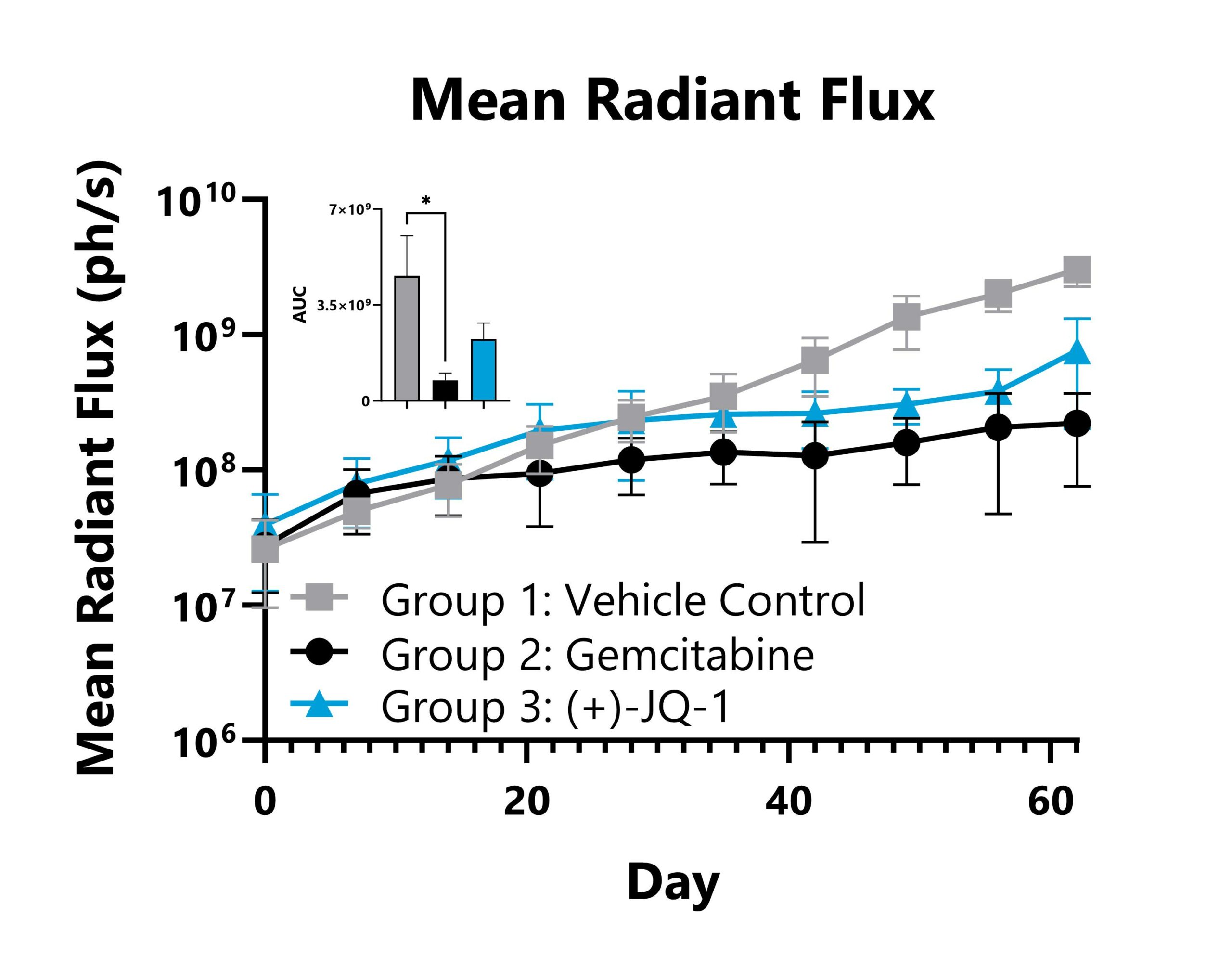
Patient-derived pancreatic cancer tumors expressing a bioluminescent reporter (PANx-005-Luc) are orthotopically seeded into immunocompromised mice. Groups 2 and 3 are subject to chemotherapy and experimental treatments, and tumor responses are measured with whole body imaging to detect luminescence. Whole body mean radiant flux values are shown with AUC analysis.

Close
Study Models

Widespread, metastatic dissemination of cancerous cells from the original tumor site is the primary cause of cancer-related mortality. As such, there is a clear need for further identification of druggable targets and the development of corresponding therapeutics to mitigate this disease progression. Murine models of cancer metastases have been developed to advance this research using tumor cells that stably express bioluminescent or fluorescent reporters. These cells are initially implanted at an orthotopic site, and metastases can then be quantifiably assessed with in-life imaging. BioModels has experience with a number of orthotopic tumor placements as well as the capabilities to quantifiably track bioluminescence or fluorescence. Representative data is shown from a patient-derived xenograft model using triple-negative breast cancer tumors that express a bioluminescent reporter as well as a syngeneic model of murine melanoma with a bioluminescent reporter.
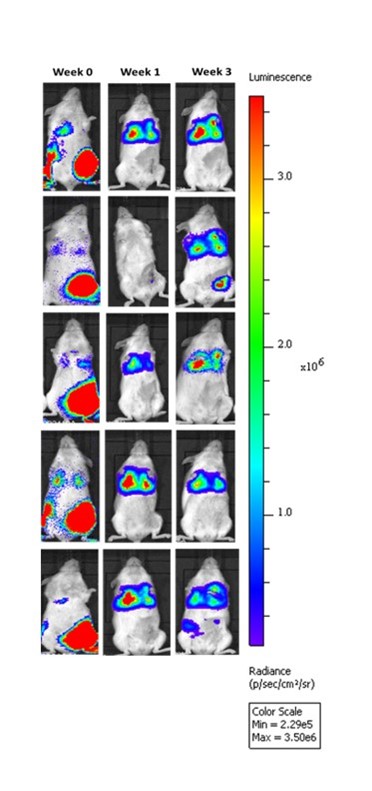
Patient derived triple-negative breast cancer tumors expressing a bioluminescent reporter (HBCx-14-Luc) are seeded into the fourth mammary fat pad in immunocompromised mice and then resected after growth criteria is met. Upon resection of the primary tumors (Week 0), treatment paradigms are introduced, and metastatic progression is monitored in the lungs of the animals via IVIS imaging to detect luminescence.
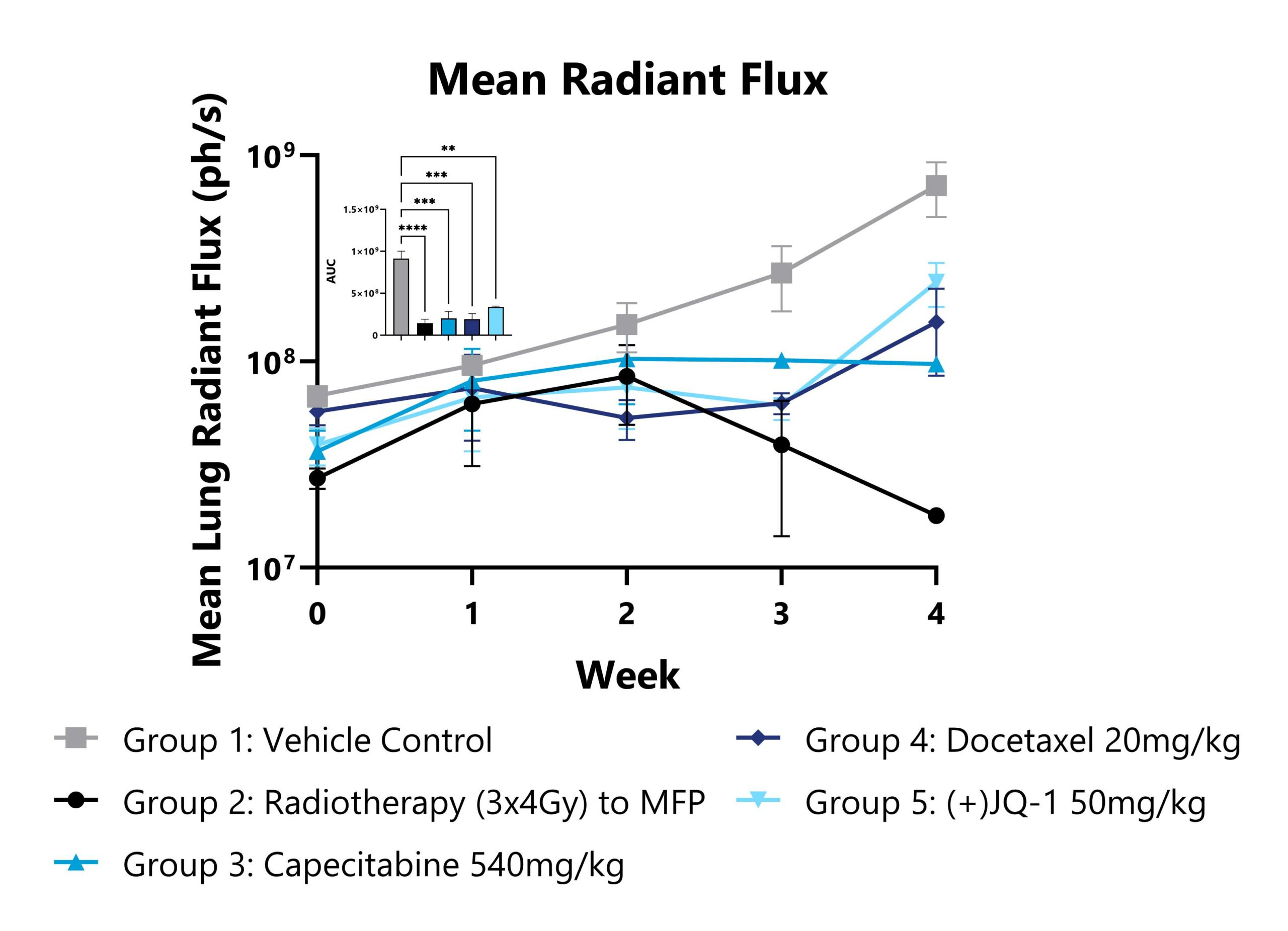
Patient derived triple-negative breast cancer tumors expressing a bioluminescent reporter (HBCx-14-Luc) are seeded into the fourth mammary fat pad in immunocompromised mice and then resected after growth criteria is met. Upon resection of the primary tumors (Week 0), treatment paradigms are introduced, and metastatic progression is monitored in the lungs of the animals via IVIS imaging to detect luminescence. Mean radiant flux values from the lung region of each group are shown (**p<0.01, ***p<0.001, ****p<0.0001).
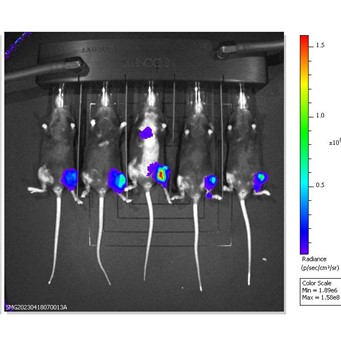
B16-F10-Luc2 cells expressing a bioluminescent reporter are seeded into the left tibia of C57Bl/6 mice to model bone metastasis of melanoma. Tumor progression is monitored via IVIS imaging to detect luminescence. Day 14 images shown.
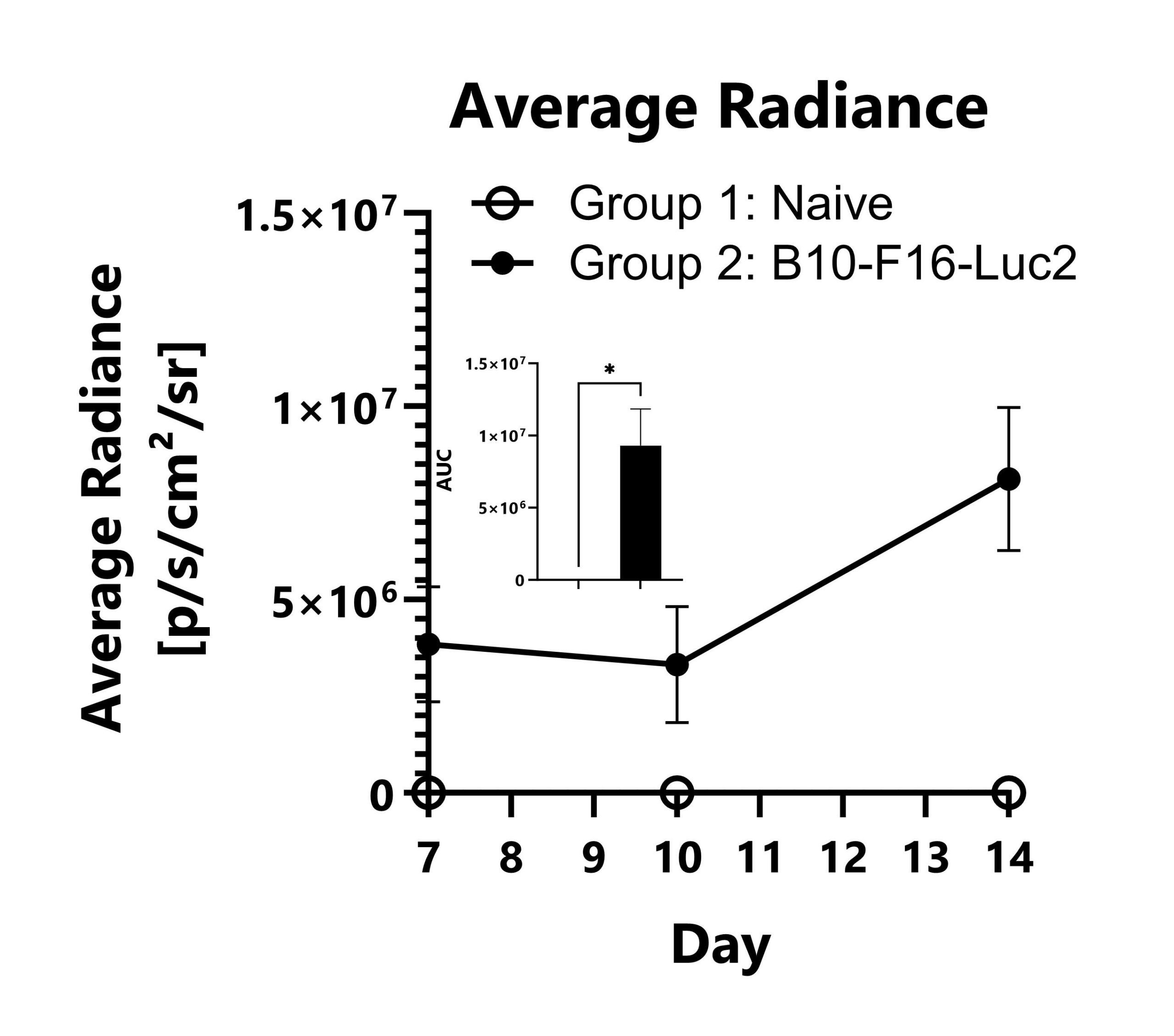
B16-F10-Luc2 cells expressing a bioluminescent reporter are seeded into the left tibia of C57Bl/6 mice to model bone metastasis of melanoma. Tumor progression is monitored in the legs of the animals via IVIS imaging to detect luminescence. Average radiance values from the leg region of each group are shown (*p<0.05).
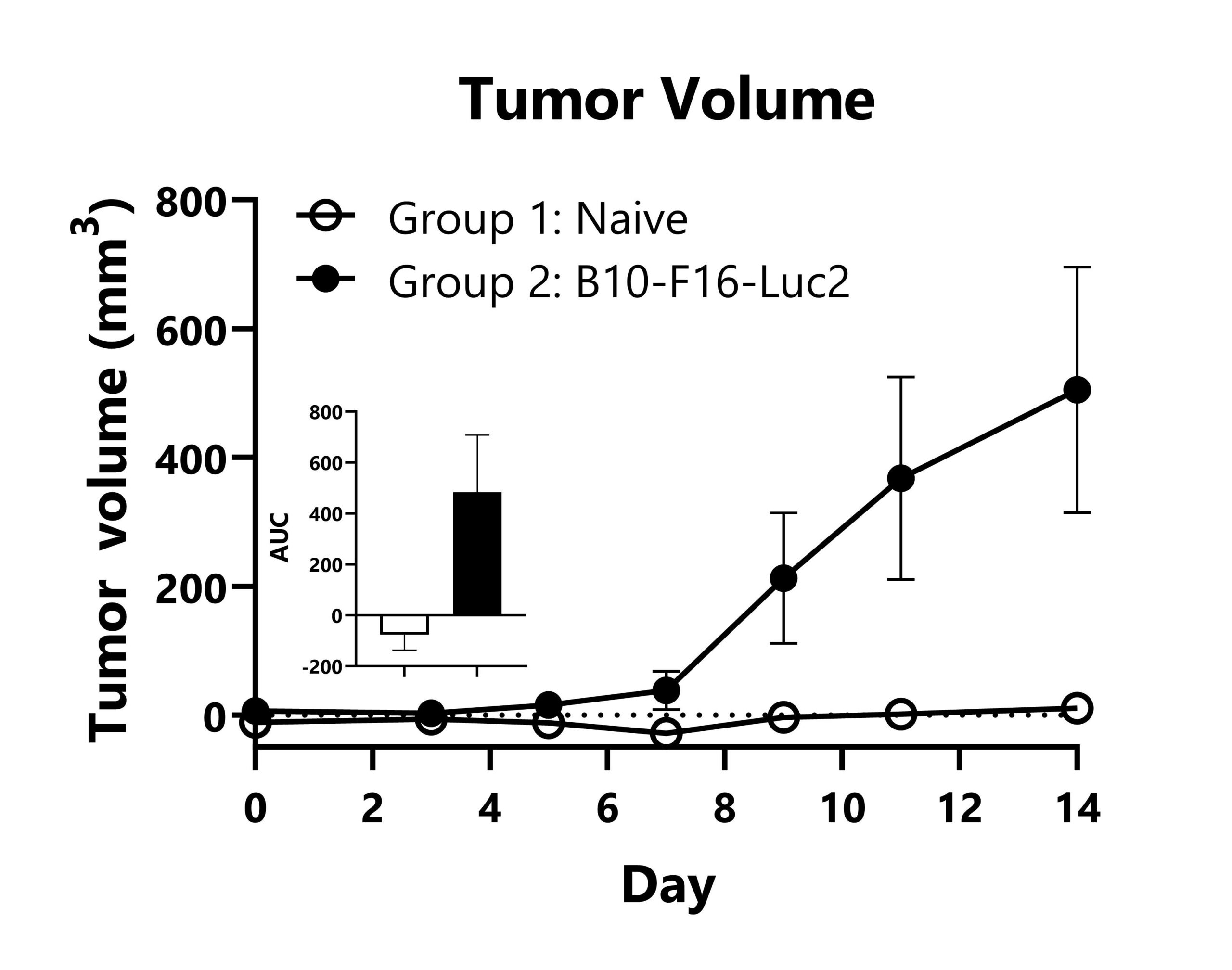
B16-F10-Luc2 cells expressing a bioluminescent reporter were seeded into the left tibia of C57Bl/6 mice to model bone metastasis of melanoma. Mean tumor volume values per group are shown with AUC analysis (*p<0.05).

Close
Study Models

The Azoxymethane/Dextran Sodium Sulfate (AOM/DSS) model of inflammation associated polyposis is a routinely utilized model that accurately recapitulates many features of human disease. Animal exposure to AOM, a carcinogen, results in a somatic inactivating mutation in a Wnt pathway kinase, leading to the generation of spontaneous cancerous colorectal polyps. The addition of DSS damages the mucosal layer, with concomitant inflammation, exacerbating the AOM-induced phenotypes. Weight loss with resolution is observed following the DSS-administration period. Polyps begin to develop approximately one week later, and colitis is observed through study duration.
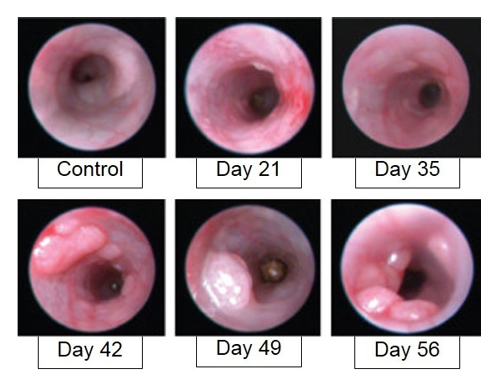
AOM/DSS-induced animals are assessed for colon inflammation and polyp development by video endoscopy.

Close