- Research Services
- Capabilities
- About Us
- Resources
- Contact Us
 Colitis and Inflammatory Bowel Diseases Models
Colitis and Inflammatory Bowel Diseases Models Graft vs Host Diseases
Graft vs Host Diseases Arthritis
Arthritis Multiple Sclerosis
Multiple Sclerosis Systemic Inflammation Models
Systemic Inflammation Models Dermatitis
Dermatitis Allergic Diseases
Allergic Diseases Additional Immune Mediated Diseases Models
Additional Immune Mediated Diseases Models Custom Models
Custom Models Inflammatory and Immune Mediated Diseases
Inflammatory and Immune Mediated DiseasesBioModels has extensive experience with a variety of animal models that capture critical mechanisms of human inflammatory and autoimmune diseases.
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established IBD/colitis models. Crohn’s Disease and Ulcerative Colitis, collectively considered IBD, are chronic intestinal disorders affecting approximately 1.4 million Americans. BioModels’ colitis models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis.
The BioModels team is experienced with numerous preclinical rodent colitis models, including chemically induced and immune-mediated models, and diverse experimental endpoints, such as:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established Graft-versus-host disease (GVHD) models. For patients undergoing stem cell transplant, GVHD is a significant cause of morbidity and mortality which presents as a heterogeneous condition involving multiple organs. BioModels’ GVHD models allow you to evaluate the immunopathology of post-transplant disease in various organs such as skin, gut, liver, and lung.
The BioModels team is experienced with multiple GVHD model induction paradigms which are available with diverse experimental endpoints, such as:
Assess your potential/novel therapeutics for efficacy and mechanism of action in BioModels’ established arthritis models. Our broad offering of rodent models of arthritis captures a spectrum of disease mechanisms and phenotypes. The BioModels team can work with you to help select the best model based on your potential targets and study goals.
Our literature-validated experimental endpoints include:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established Experimental Autoimmune Encephalomyelitis (EAE) models of Multiple Sclerosis (MS). MS, the most common neurological disease of young adults, is an inflammatory, T-cell mediated autoimmune disease of the central nervous system (CNS). BioModels’ EAE models are T cell mediated, and stimulation of an immune responses against CNS antigens is the underlying mechanism of the model.
The BioModels team is experienced with both the MOG induced model of chronic EAE as well as the PLP model of relapsing EAE; both are available with diverse endpoint assessment such as:
Assess your novel therapeutics for efficacy and mechanism of action in BioModels’ established systemic inflammation models. BioModels’ systemic inflammation models allow you to evaluate acute inflammatory responses in vivo.
The BioModels team is experienced with a range of inflammation induction pathways which are available with diverse experimental endpoints, such as:
Assess your novel therapeutics for efficacy and mechanism of action in our established dermatitis models. Dermatitis is an umbrella term for a group of conditions that cause inflammation of the skin. The etiology for these conditions can be atopic (allergic), environmental, or a side effect of radiation therapy used to treat some forms of cancer. We offer several rodent models of dermatitis that are well-established and capture a spectrum of disease mechanisms and phenotypes.
Our literature-validated experimental endpoints include:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established allergic disease models. Allergic diseases, such as allergic asthma, food allergy, and eosinic esophagitis, are conditions in which the immune system overreacts to innocuous environmental antigen (allergen). BioModels’ allergic disease models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis.
The BioModels team is experienced with numerous preclinical rodent allergic disease models, including acute and chronic models that utilize various common allergens, and diverse experimental endpoints, such as:
Immune-mediated inflammatory disease describes a diverse grouping of conditions with both localized and systemic inflammation, typically with multi-organ involvement. These multifactorial diseases characterized by immune dysregulation may present with pain, chronic fatigue, skin manifestations, localized inflammation, and often affect the patient’s quality of life. At BioModels, assess your potential/novel therapeutics for efficacy and mechanism of action in a variety models.
Our literature-validated experimental endpoints include:
The pace of drug discovery and preclinical research is rapidly increasing and ever evolving. BioModels frequently collaborates on proof-of-concept experiments designed to validate novel targets and approaches that have been described in the literature. While our internally optimized disease models are suitable for many projects, we understand that each program is unique, and it’s our goal to provide custom solutions to our clients. We have a history of success with custom modeling approaches for inflammatory and immune mediated diseases and are always looking for ways to expand our repertoire.
The BioModels team encourages detailed discussions and consultation aimed at identifying the most appropriate study designs for the models of interest. We are very experienced with the replication of published data to support our clients’ preclinical programs and are well-versed in the troubleshooting approaches that often arise when adapting literature-based methodologies to suit research needs.
Study Models

The DSS model is among the most published IBD models in the field, and is frequently used in drug development. It presents with neutrophilic immune infiltrate and compromised mucosal barrier integrity. DSS can be utilized in mice or rats depending on experimental needs. Colitis is induced via exposure to DSS-treated drinking water. In mice, weight loss is observed thereafter and depending on the study duration, can ultimately recover and return to baseline. In rats, a reduction in weight gain is typically observed vs. overt weight loss. Colitis remains robust through the experimental duration and studies can be terminated during the acute or semi-chronic phase of the model. In mice, test article responses can be compared to the effects of Anti-IL-12-p40 mAb.
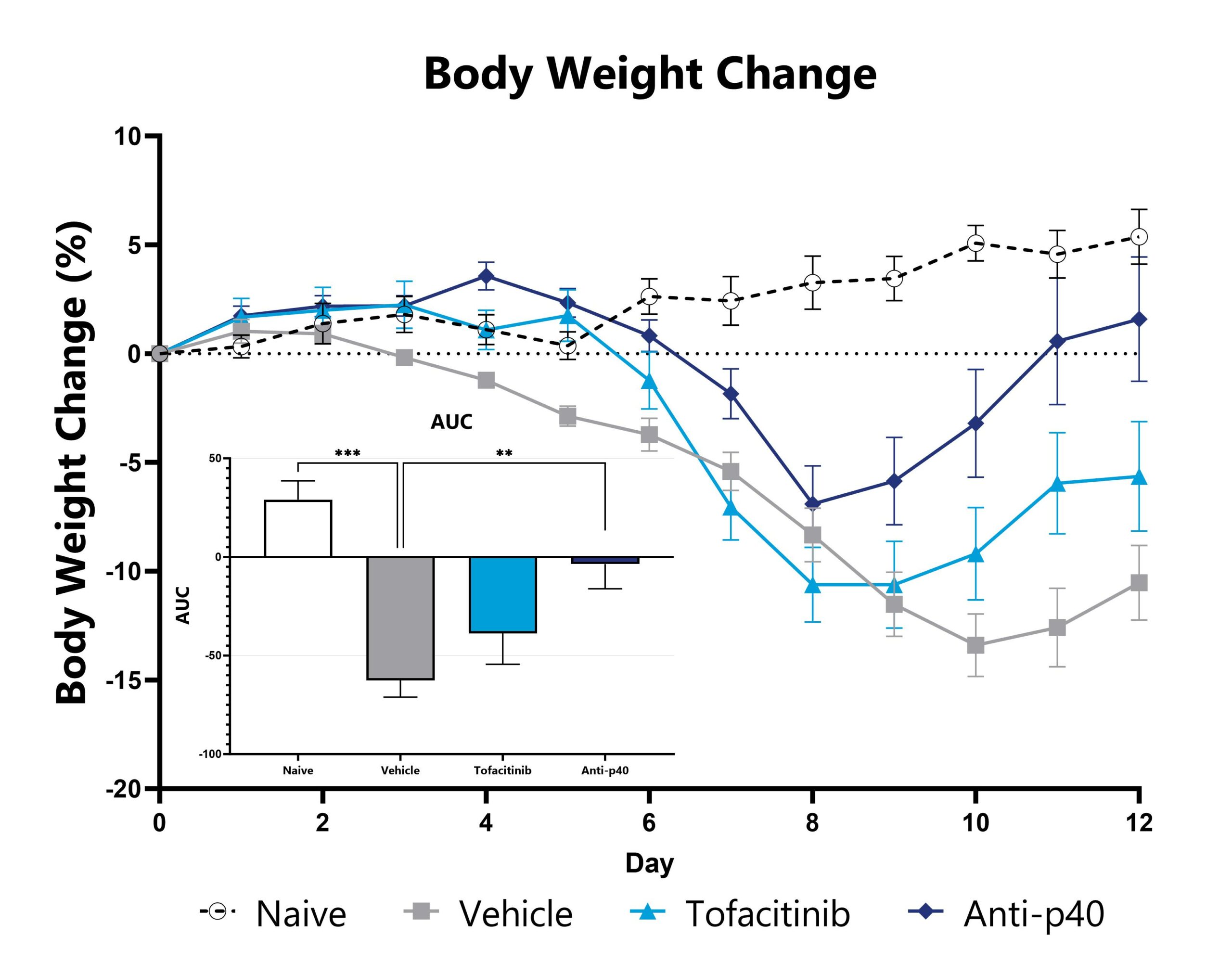
DSS-induced animals are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (**p<0.01; ***p<0.005 compared to the vehicle-control).
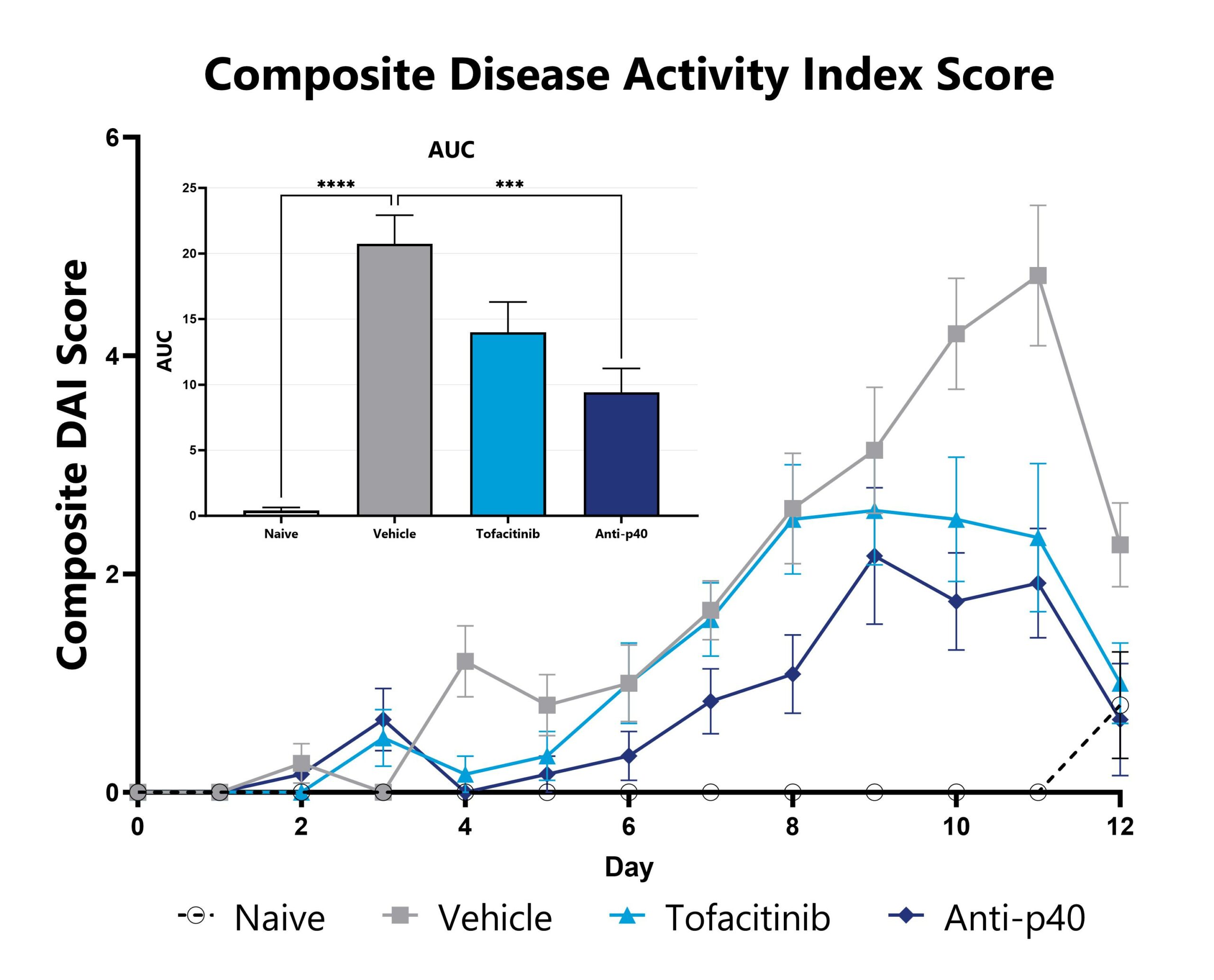
DSS-induced animals are assessed daily for weight loss, diarrhea, and blood in stool phenotypes. Each phenotype is scored and used to calculate the composite Disease Activity Index score. The AUC is calculated to compare treatment arms and is shown in the inset. (***p<0.005 compared to the vehicle-control).
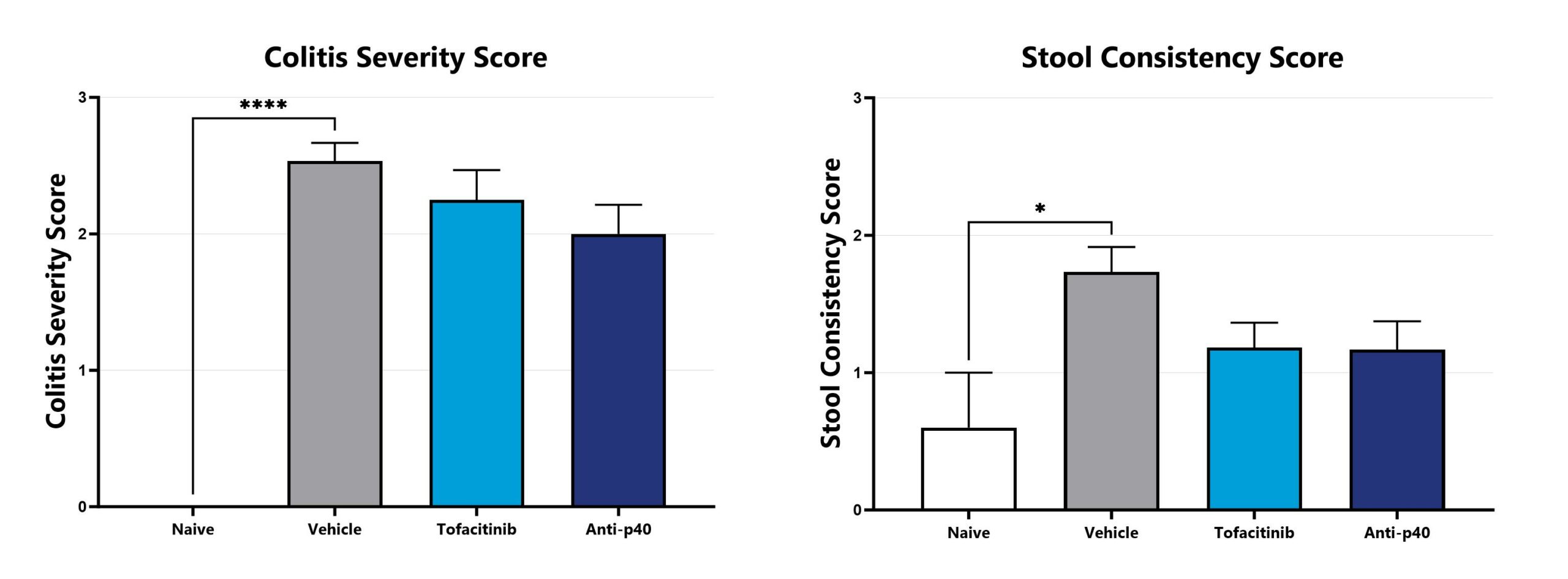
DSS-induced animals are assessed for colonic inflammation and ulceration by video endoscopy and are scored for colitis severity (left) and stool consistency (right). (*p<0.05; ****p<0.001 compared to the vehicle-control).
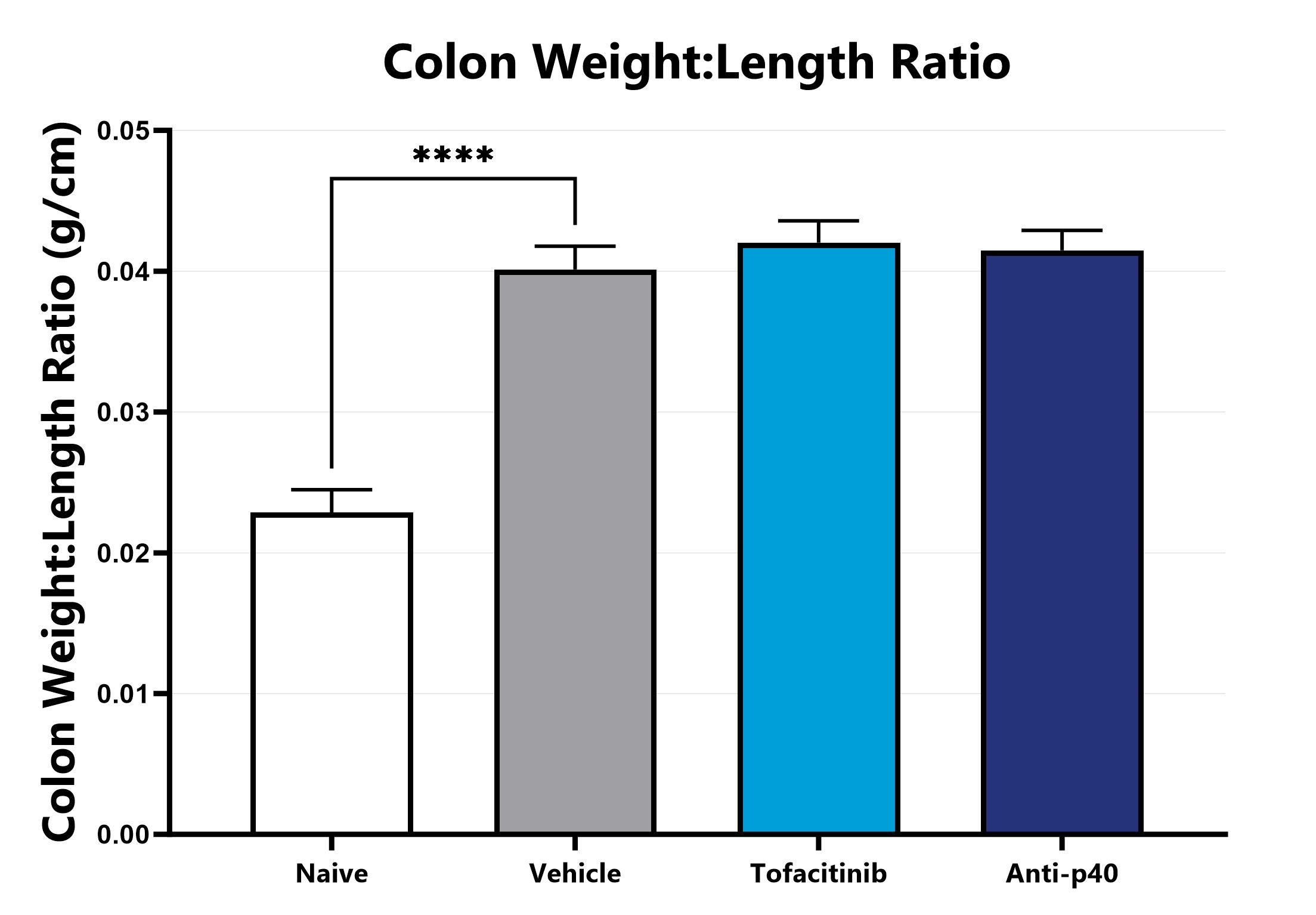
Following euthanasia, the colon is excised, measured, rinsed, and weighed; the colon weight:length ratio is calculated. (****p<0.001 compared to the vehicle-control).
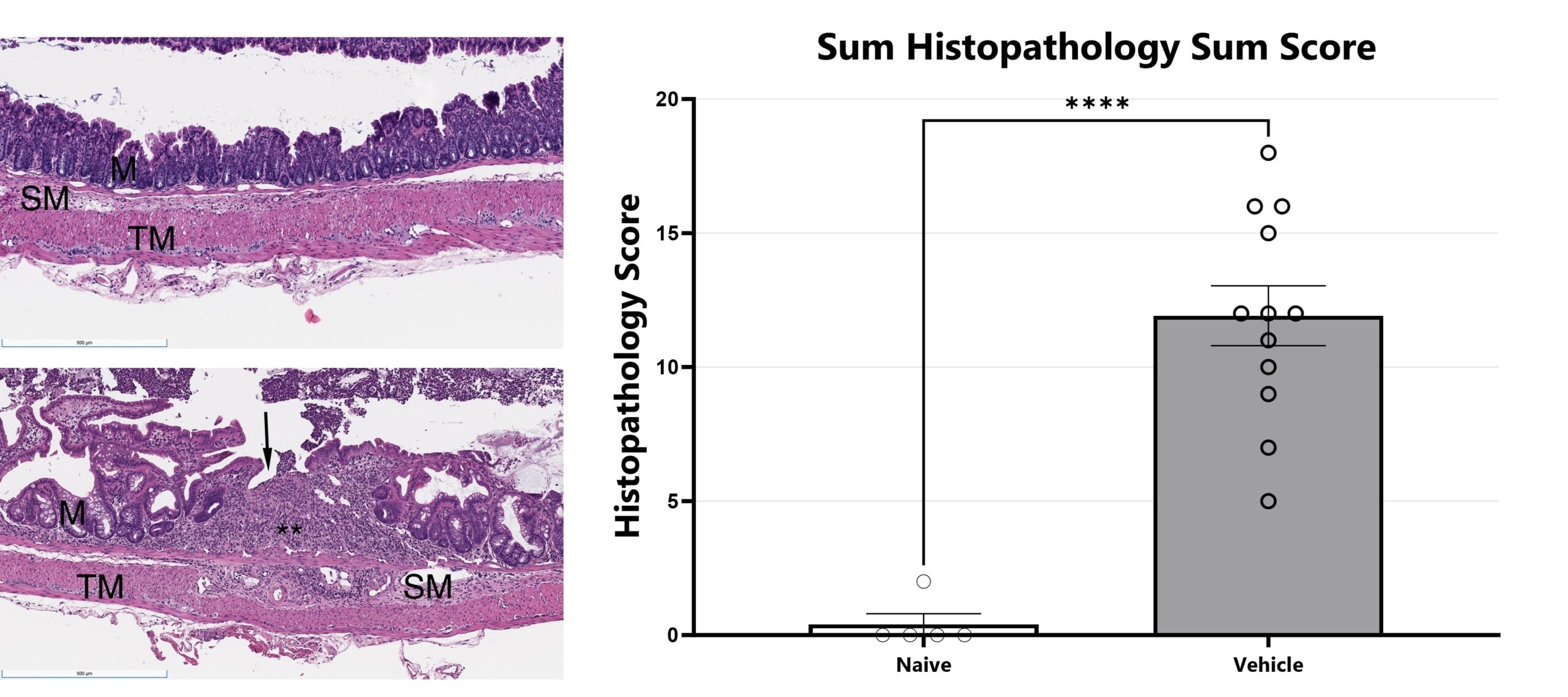
H&E stained slides from mouse colon tissue are evaluated with light microscopy by an ACVP board-certified veterinary pathologist (Dallas Tissue Research). Features of inflammation, necrosis/gland loss, erosions, submucosal edema, and epithelial hyperplasia are given an individual score and scores are added together to obtain a sum score. Naïve is shown in upper left and diseased is shown in lower left. (****p<0.001).
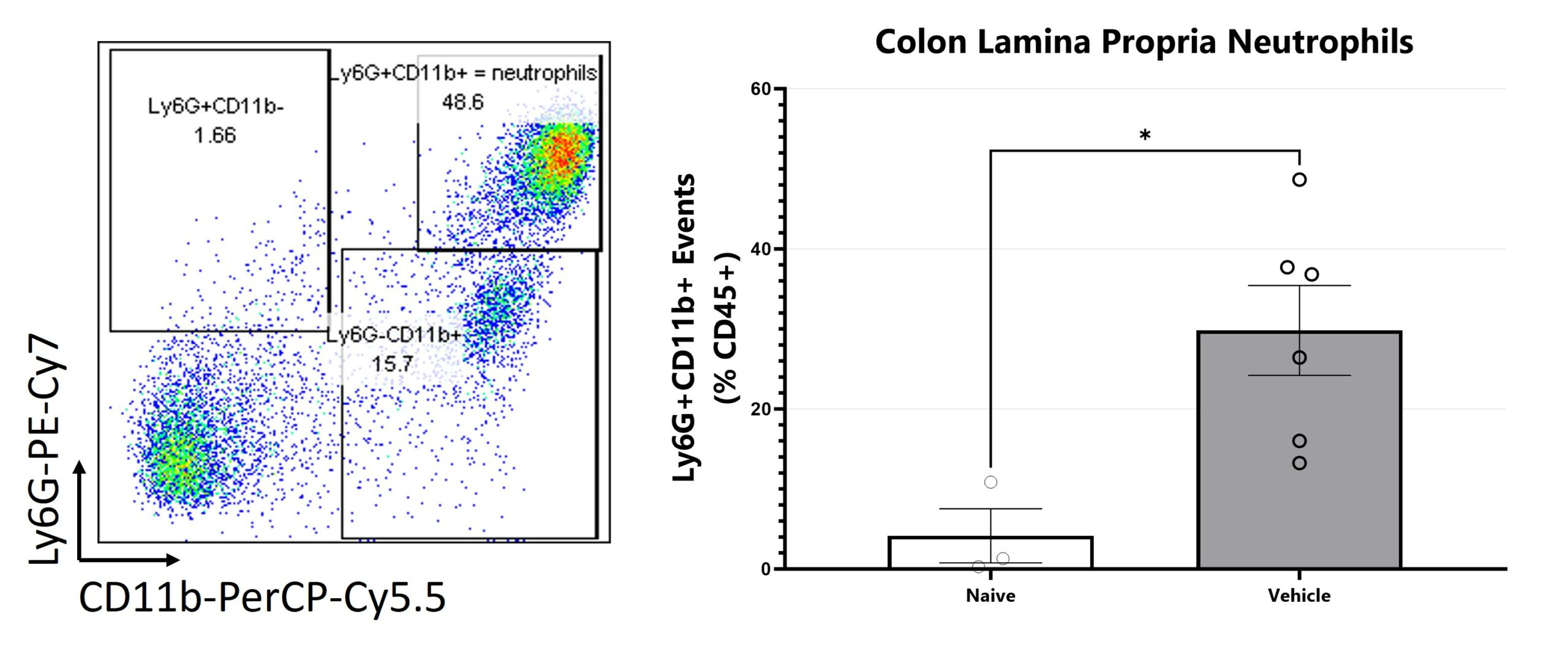
Colon samples are digested for leukocyte enrichment, stained, and are assessed via Flow Cytometry for neutrophils. (*p<0.05).
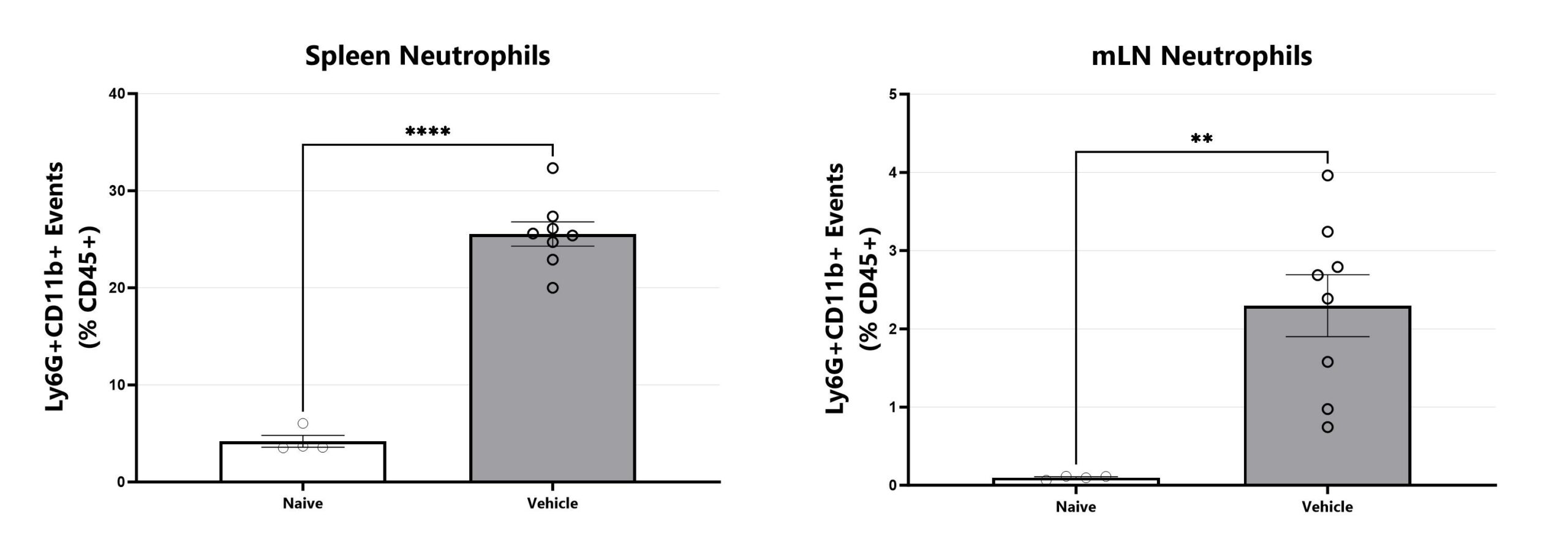
Spleen and mLN samples are dissociated, stained, and assessed via Flow Cytometry for neutrophils. Analysis for additional cell types is available. (**p<0.01; ****p<.0001).
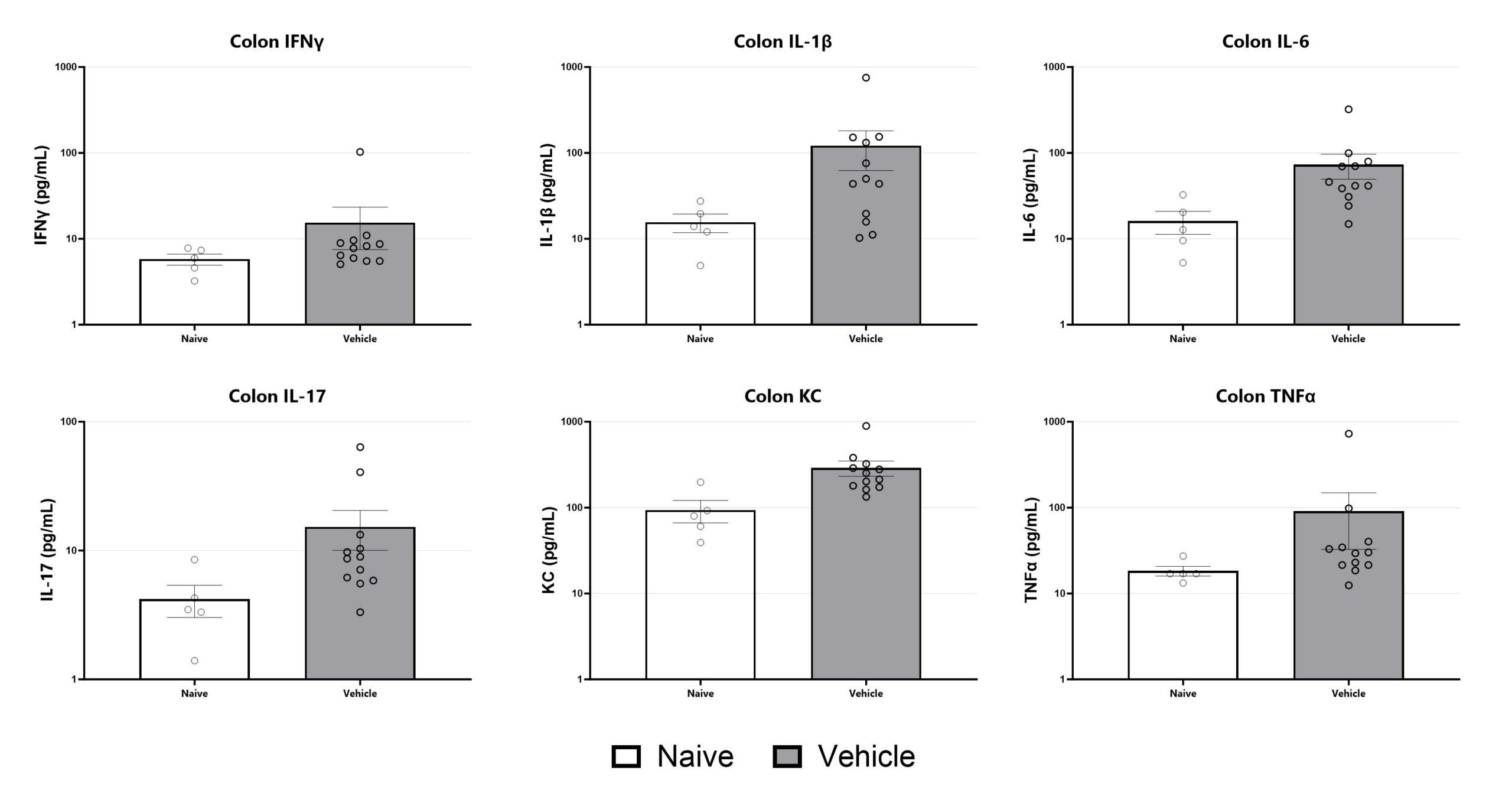
Colon samples are homogenized and assessed via multiplex cytokine analysis for IFNγ, IL-1β, IL-6, IL-17, KC, and TNFα. Additional cytokines are available.

Close

The chronic DSS model, in which animals are administered DSS over the course of three on/off cycles, is helpful for experiments requiring model chronicity. Weight loss is observed following each DSS administration period, with recovery during the off-cycle. Colitis remains robust through the experimental duration.
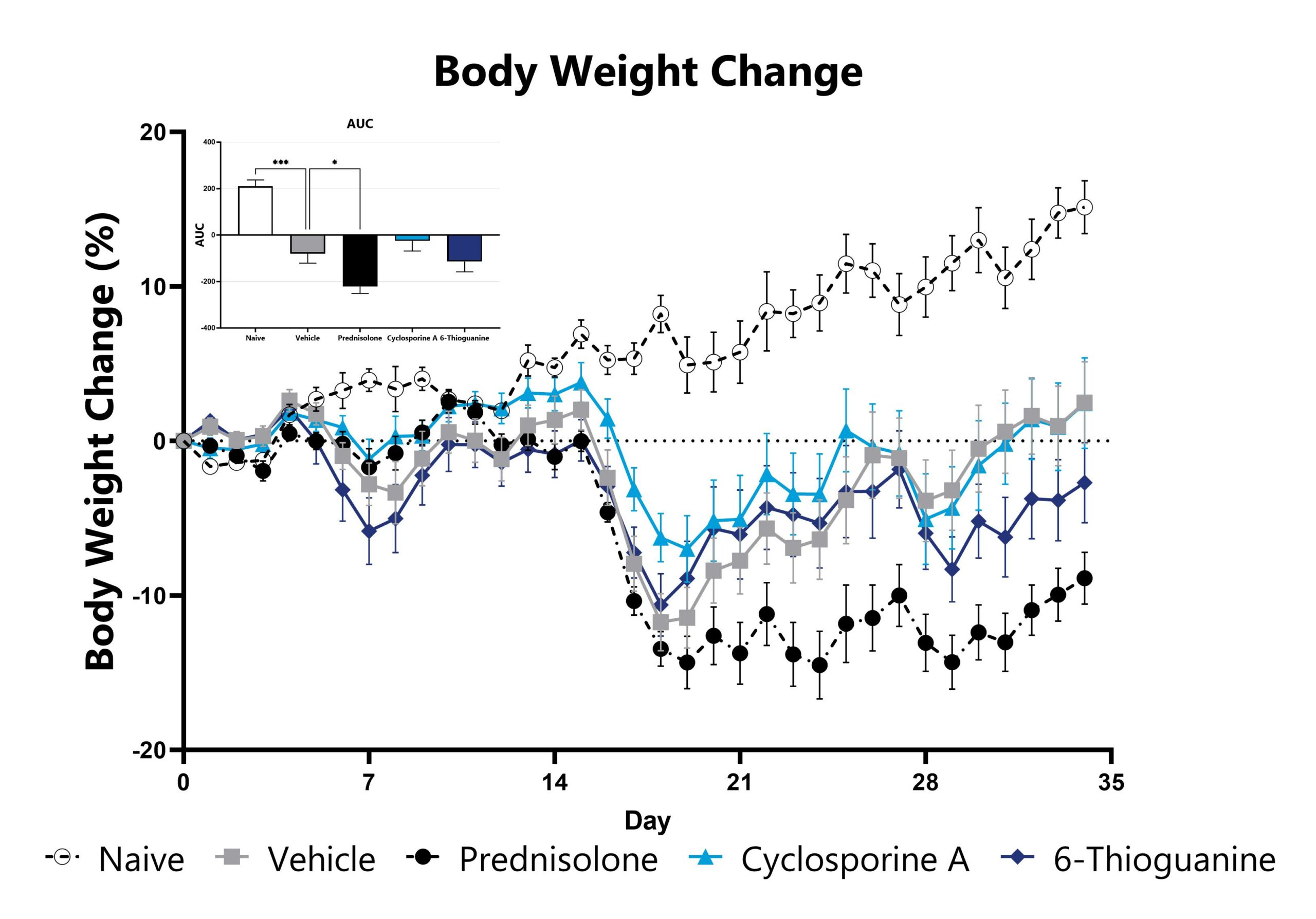
DSS-induced animals are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05; ***p<0.005 compared to the vehicle-control).
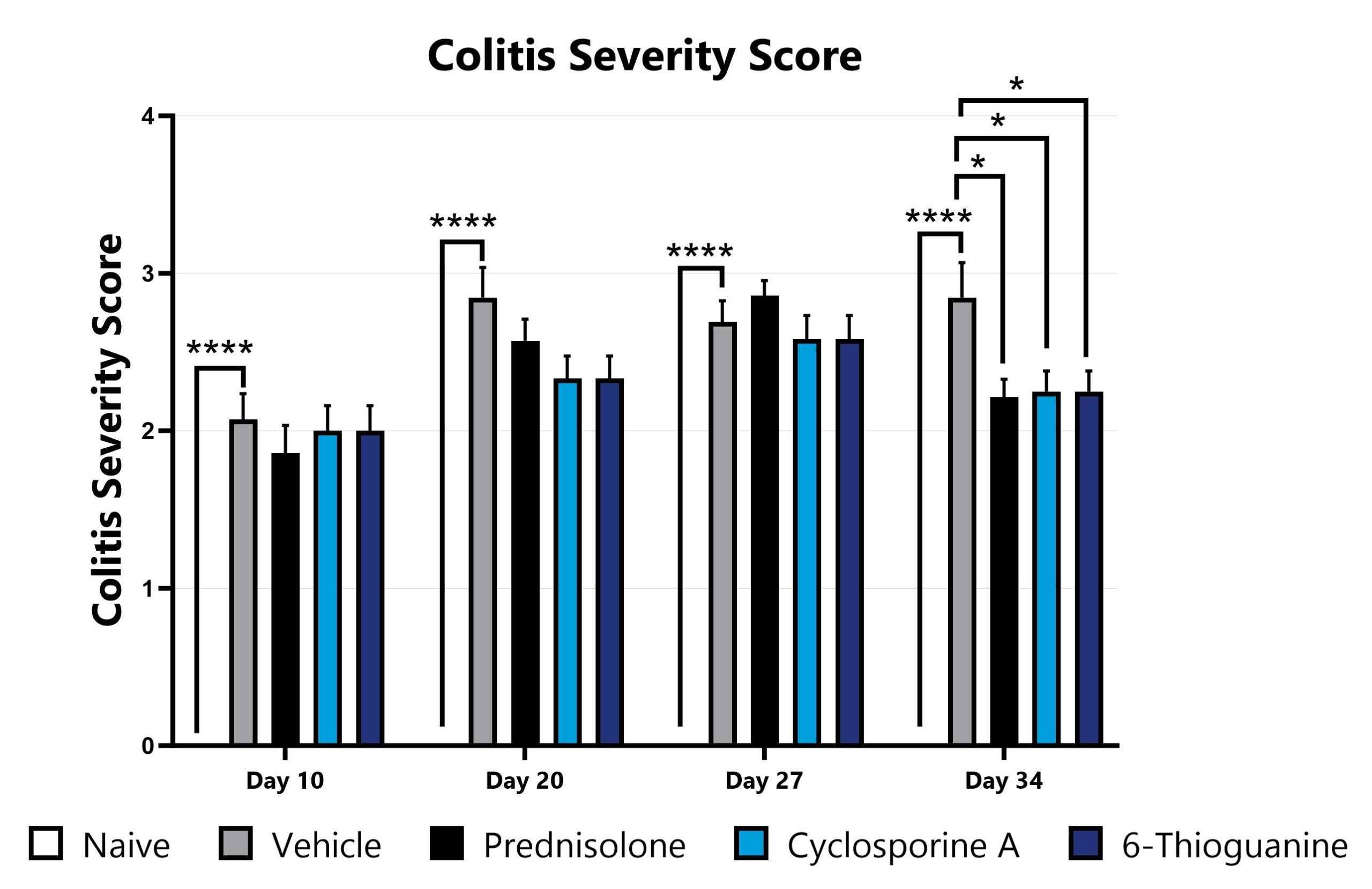
DSS-induced animals are assessed for colonic inflammation and ulceration by video endoscopy and are scored for colitis severity. (*p<0.05; ****p<0.001 compared to the vehicle-control).
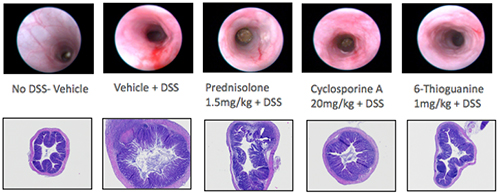
H&E-stained slides from mouse colon tissue are evaluated with light microscopy by an ACVP board-certified veterinary pathologist.

Close

TNBS is another widely published chemically-induced model of IBD in mice and rats. In the acute model, disease is induced by a single intrarectal dose of TNBS under isoflurane anesthesia. In both mice and rats, weight loss relative to non-diseased animals is observed after TNBS administration with a gradual recovery thereafter. Colitis peaks early and presents with mild recovery as the model continues.
Animals with TNBS-induced colitis are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset.

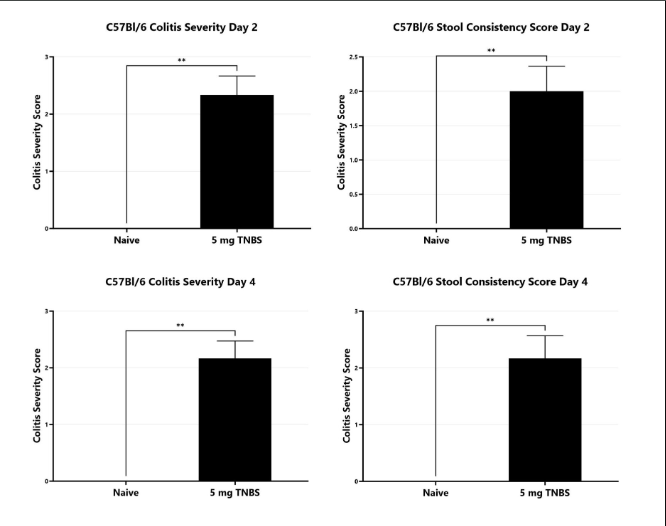
TNBS-induced animals are assessed for colonic inflammation and ulceration by video endoscopy and are scored for colitis severity (left) and stool consistency (right). (**p<0.01).
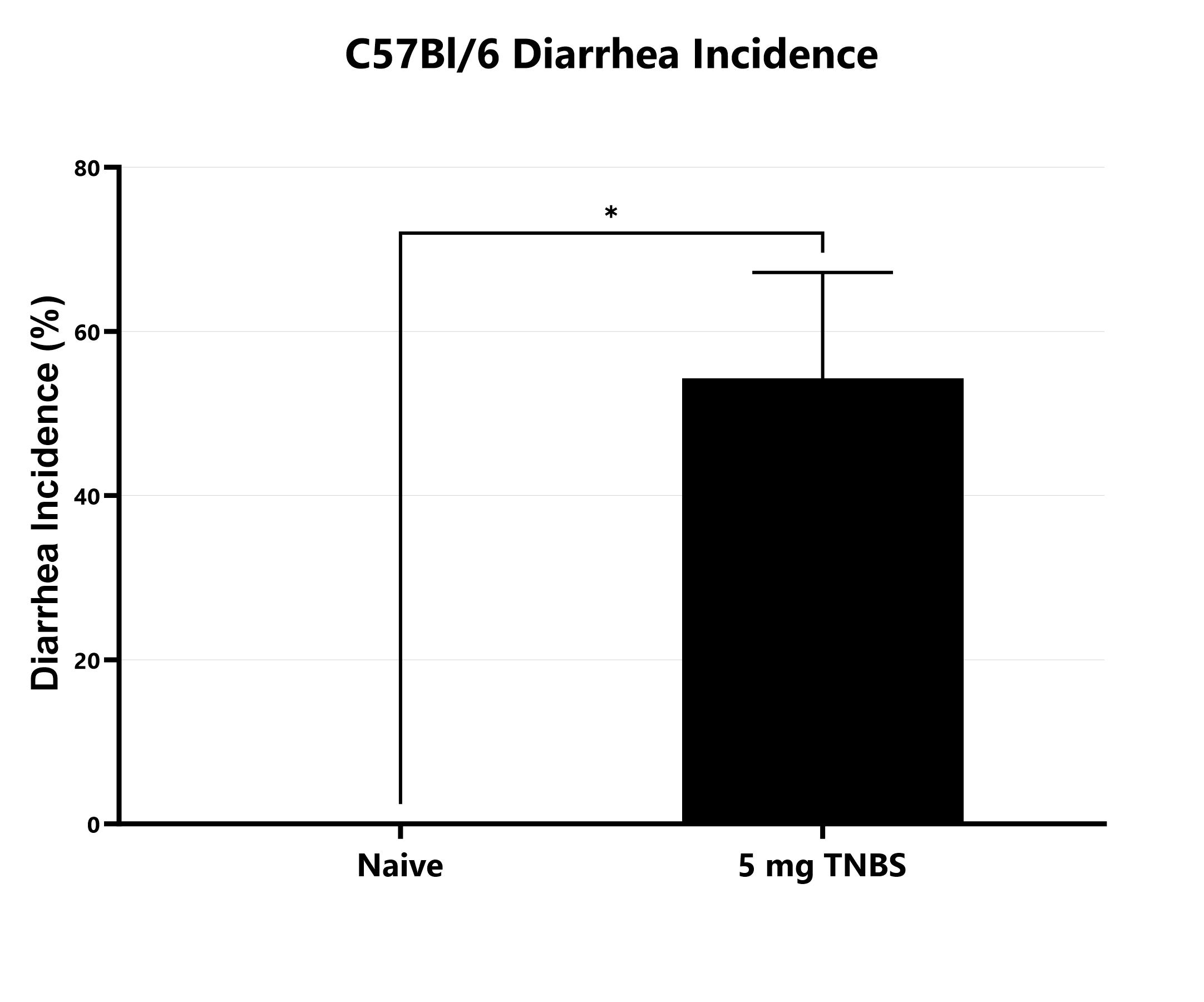
The incidence of diarrhea is monitored daily in the C57Bl/6 TNBS colitis model and presented as a percentage of days with diarrhea (*p<0.05).
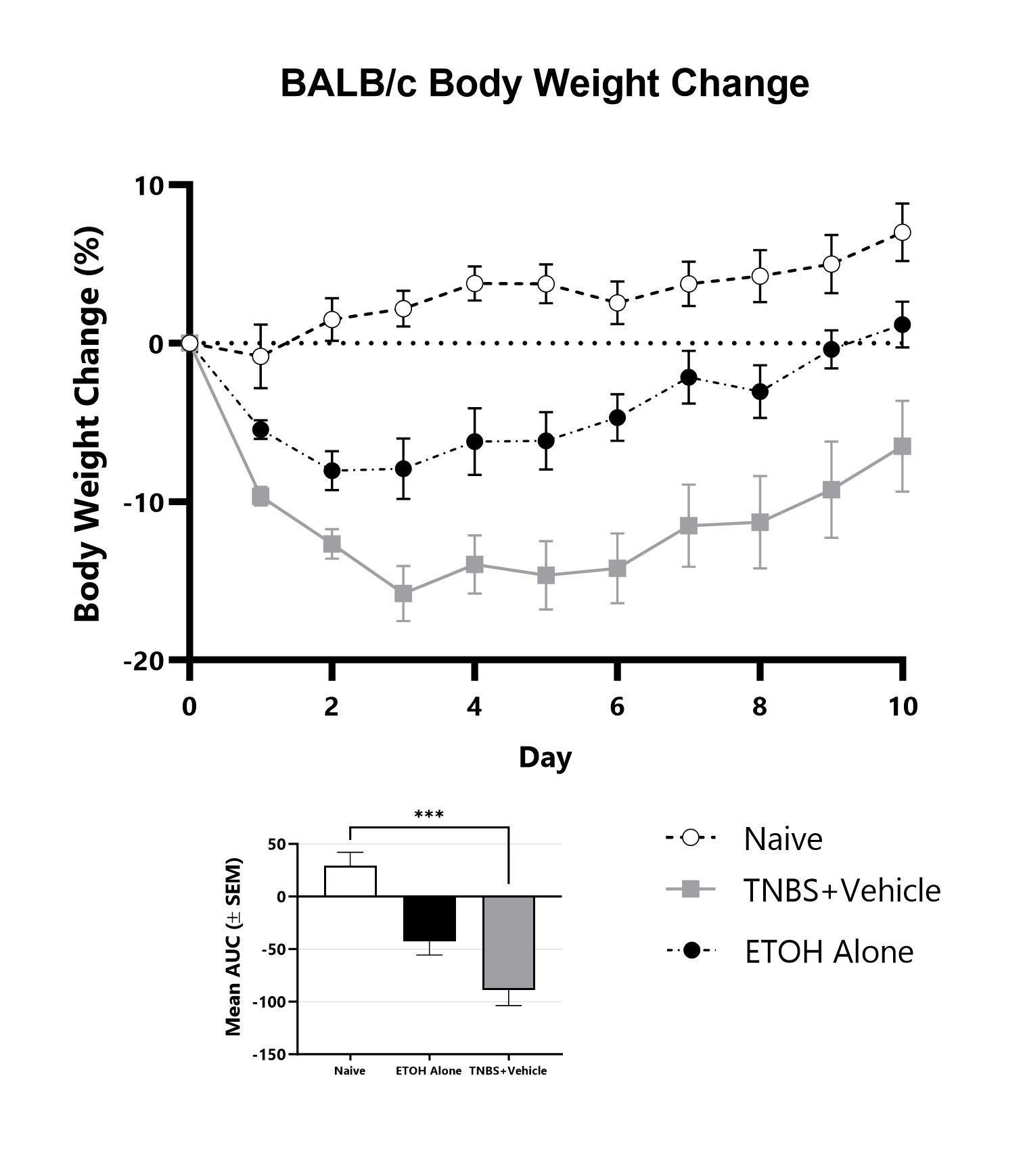
Animals with TNBS-induced colitis are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (***p<0.001 compared to the vehicle-control).
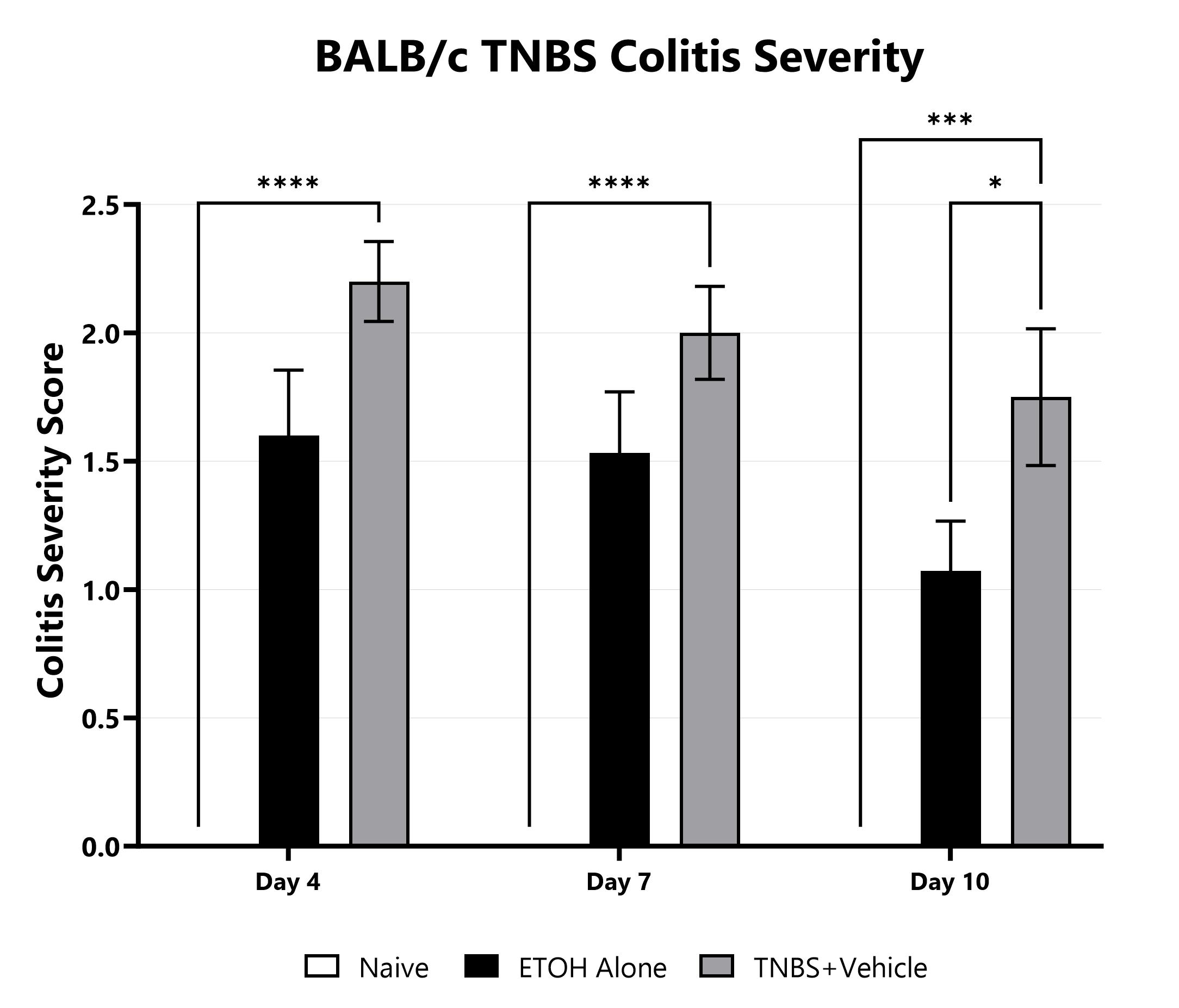
TNBS-induced animals are assessed for colonic inflammation and ulceration by video endoscopy and are scored for colitis severity. (*p<0.05; ***p<0.001; ****p<0.0001 compared to the vehicle-control).
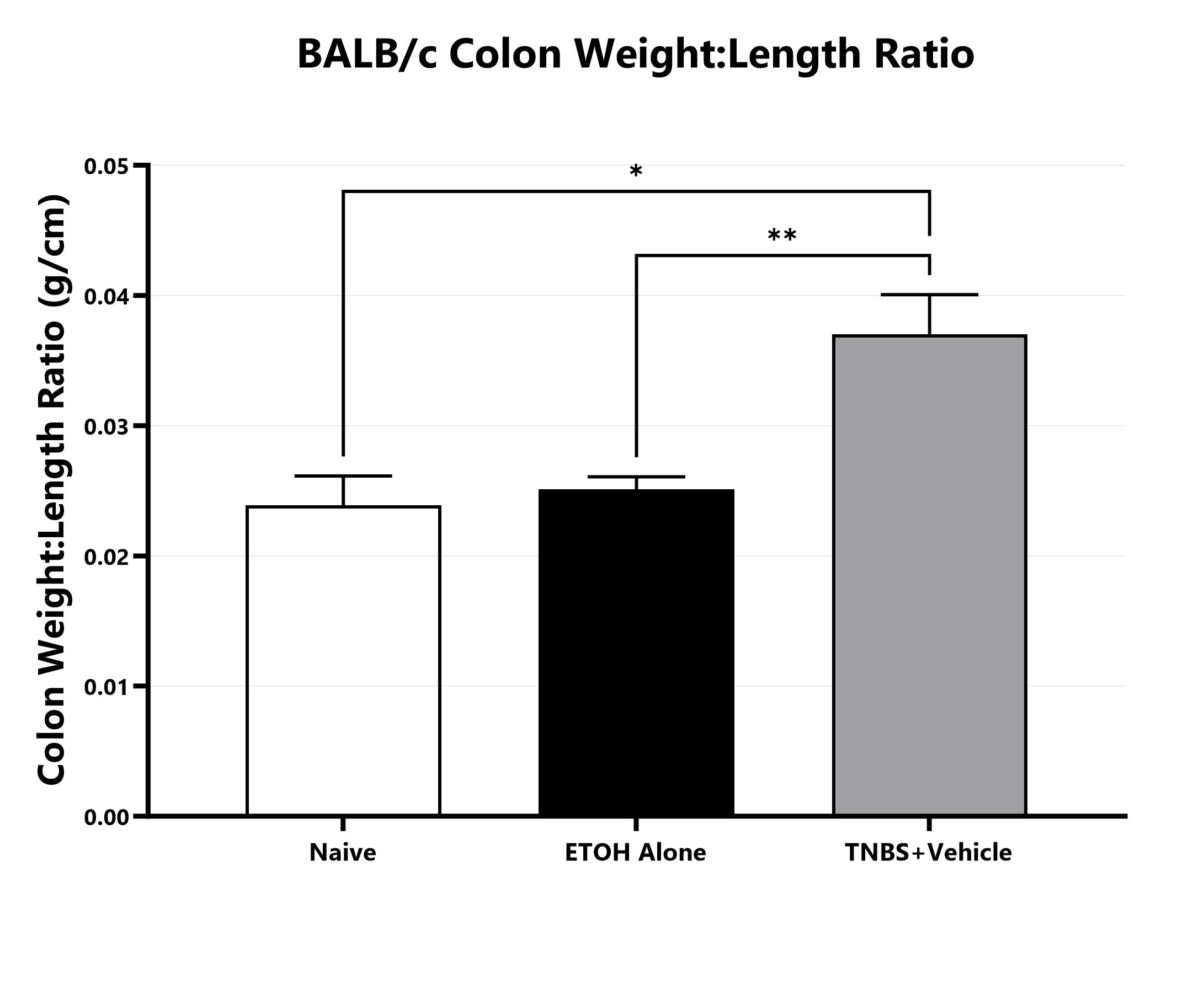
Following euthanasia, the colon is excised, measured, rinsed, and weighed; the colon weight:length ratio is calculated. (*p<0.05; **p<0.01) compared to the vehicle-control).
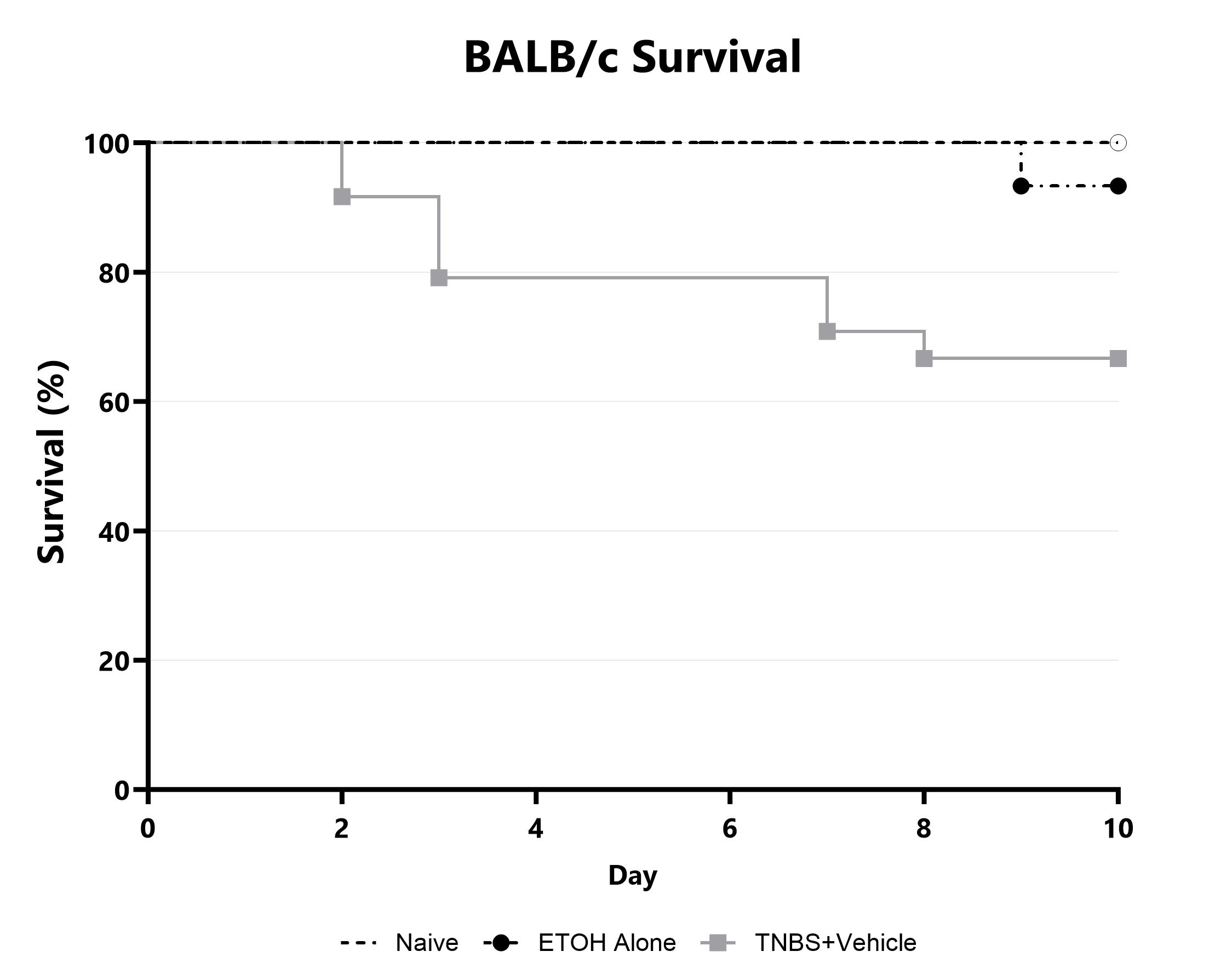
Animals are assessed for survival and moribundity on a daily basis and survival statistics (Kaplan-Meier) are determined.

Close

Oxazolone-induced colitis is another model of chemically-induced ulcerative colitis. When administered intrarectally, OXZ induces Th2-mediated acute colitis presenting in the distal colon. The model is predominantly performed in mice species such as BALB/c and SJL. Disease is induced with or without dermal OXZ pre-sensitization followed by intrarectal administration of OXZ formulated in ethanol. Body weight loss is observed following intrarectal administration of OXZ, with some body weight recovery observed by study termination. Phenotypes of the model typically include hemorrhagic inflammation, epithelial barrier disruption, and histological changes in the distal colon.
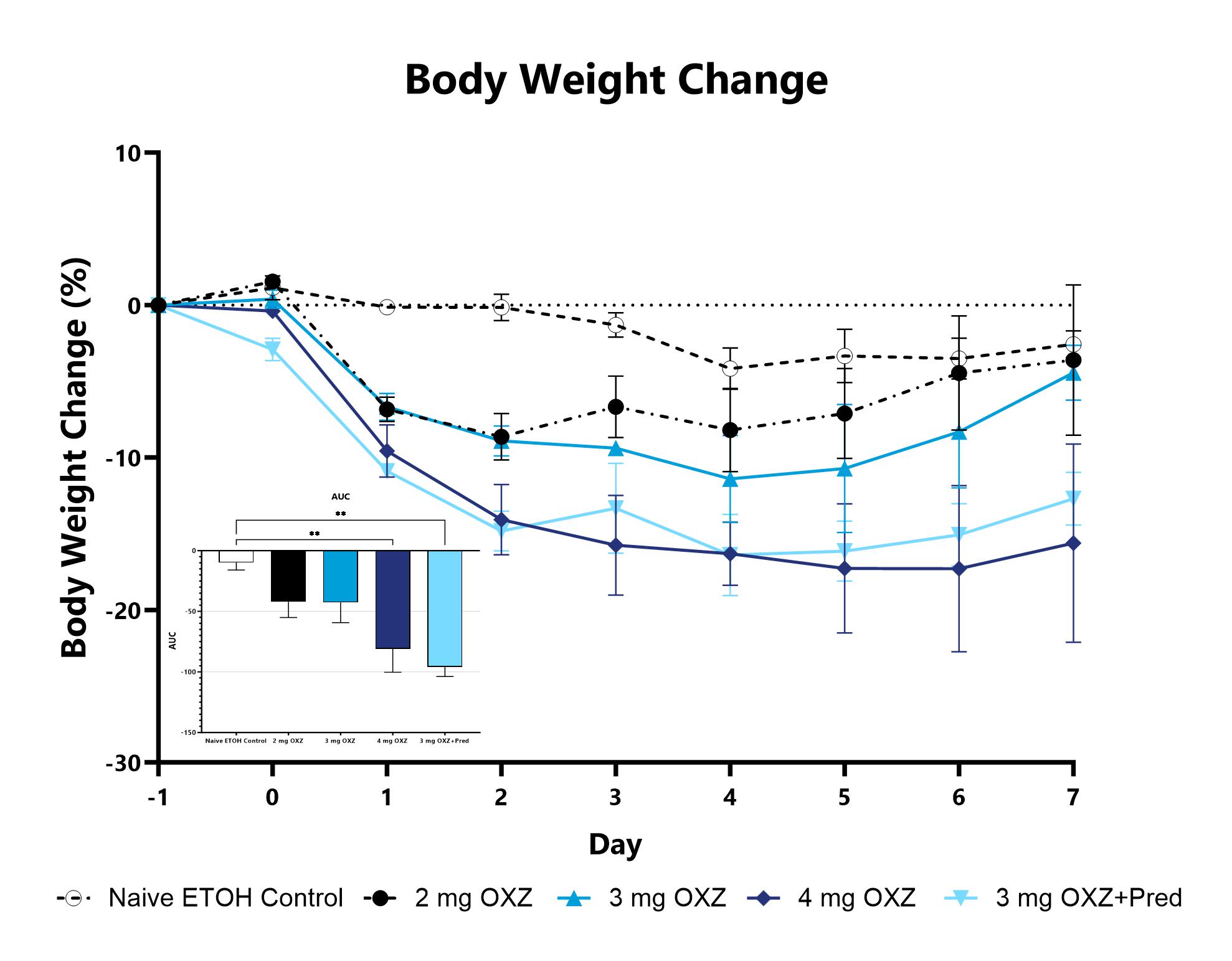
Animals with OXZ-induced colitis are weighed daily, and body weight change as compared to baseline is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (**p<0.01 compared to the Naive).
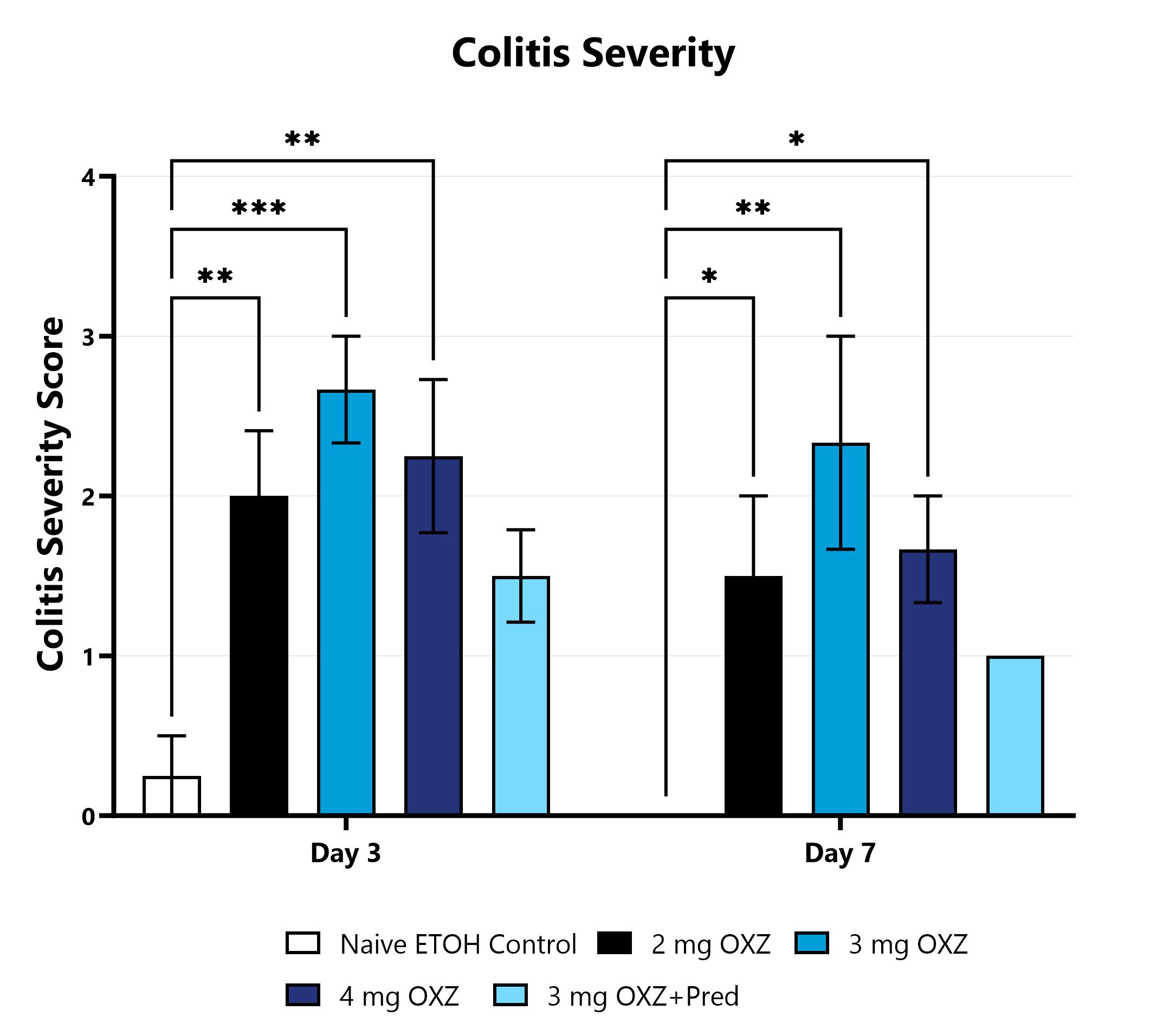
OXZ-induced animals are assessed for colonic inflammation and ulceration by video endoscopy and are scored for colitis severity. (*p<0.05; **p<0.01; ***p<0.001 compared to the Naive).

Close

The murine anti-CD40 colitis model of IBD is routinely used in drug development for experiments requiring a macrophage-mediated mechanism of action, but not requiring lymphocytes. It presents with an innate inflammatory response that is dependent on TNFα, IFNγ, and IL-12/IL-23 p40 secretion and is characterized by a systemic and local inflammatory disease. Colitis is induced via administration of an agonistic CD40 monoclonal antibody to T and B cell-deficient RAG2-/- mice. Weight loss is observed thereafter and depending on the study duration, can ultimately recover and return to baseline. Test article responses can be compared to the effects of either anti-TNF or anti-p40 mAb.
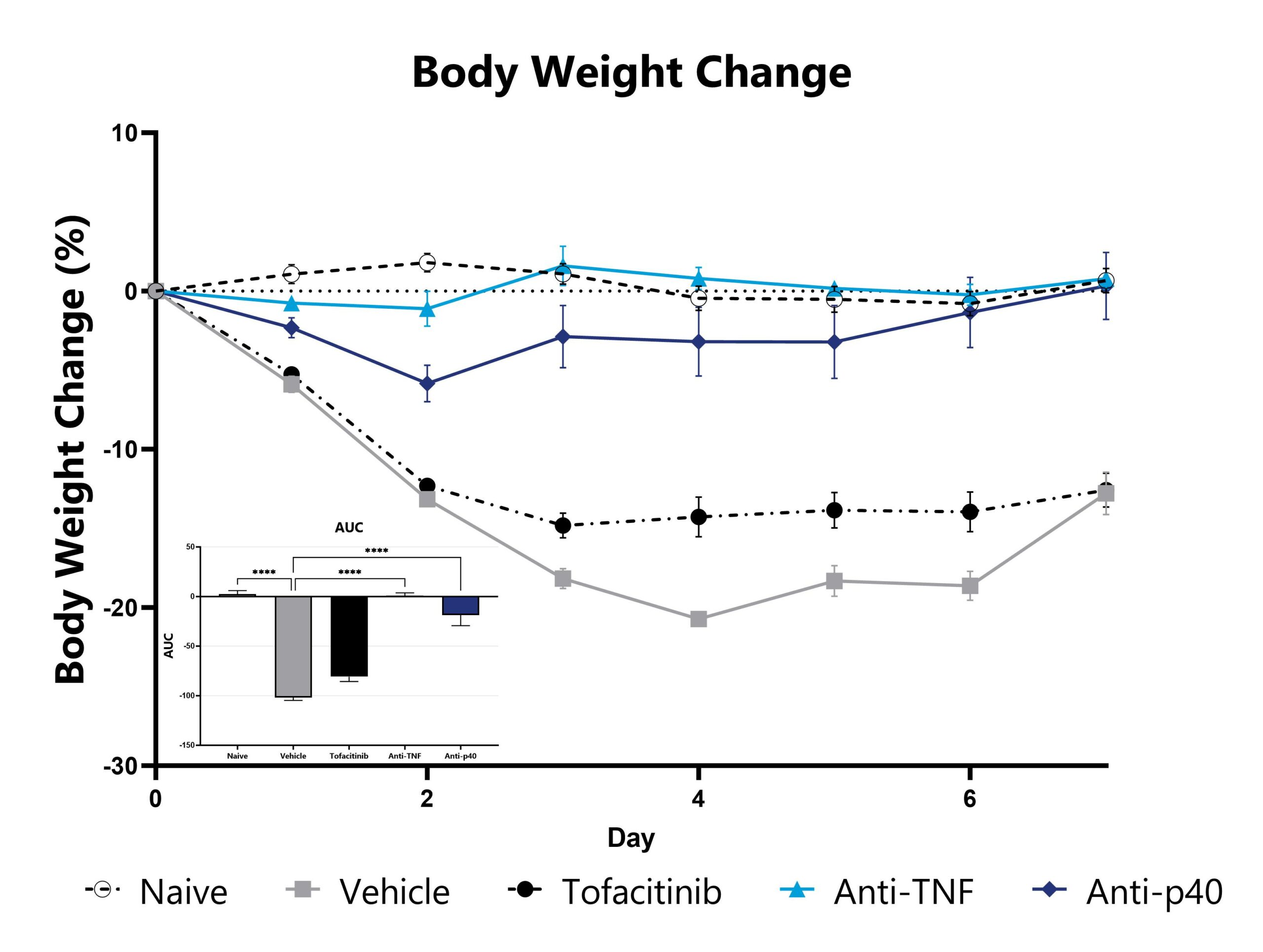
CD40 mAb-induced animals are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. ( ***p<0.005 compared to the vehicle-control).
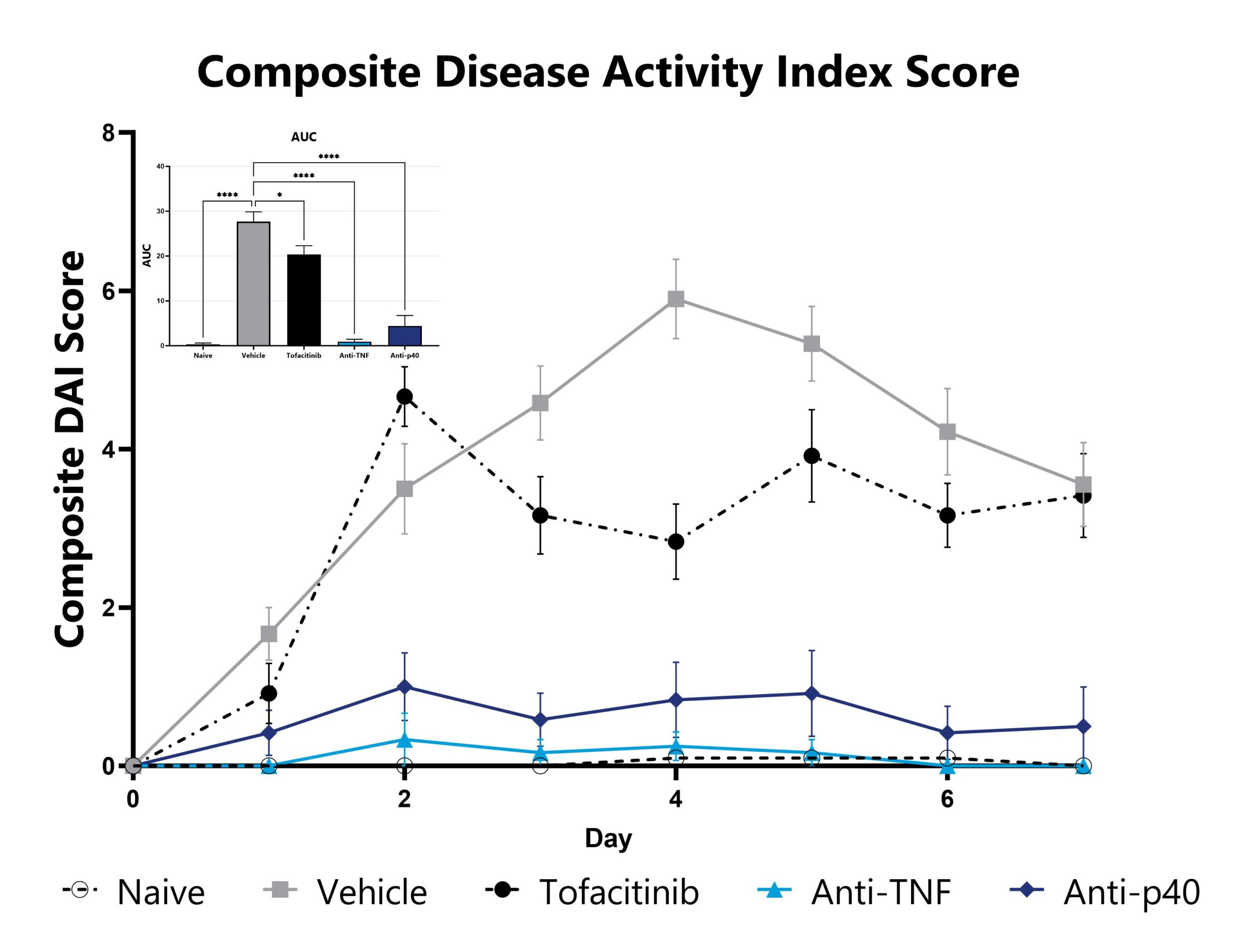
CD40 mAb-induced animals are assessed daily for weight loss, diarrhea, and blood in stool phenotypes. Each phenotype is scored and used to calculate the composite Disease Activity Index score. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05; ****p<0.001 compared to the vehicle-control).
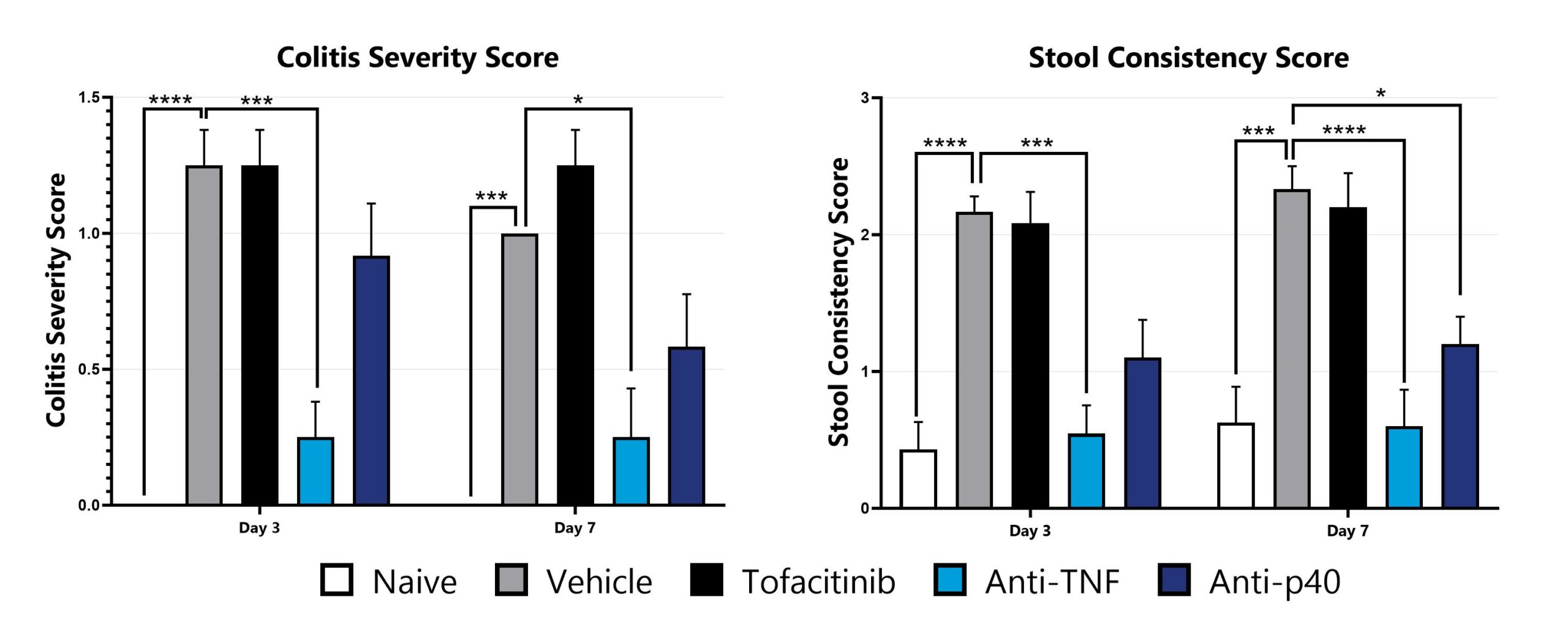
CD40 mAb-induced animals are assessed for colonic inflammation and ulceration by video endoscopy and are scored for colitis severity (left) and stool consistency (right). (*p<0.05; ***p<0.005; ****p<0.001 compared to the vehicle-control).
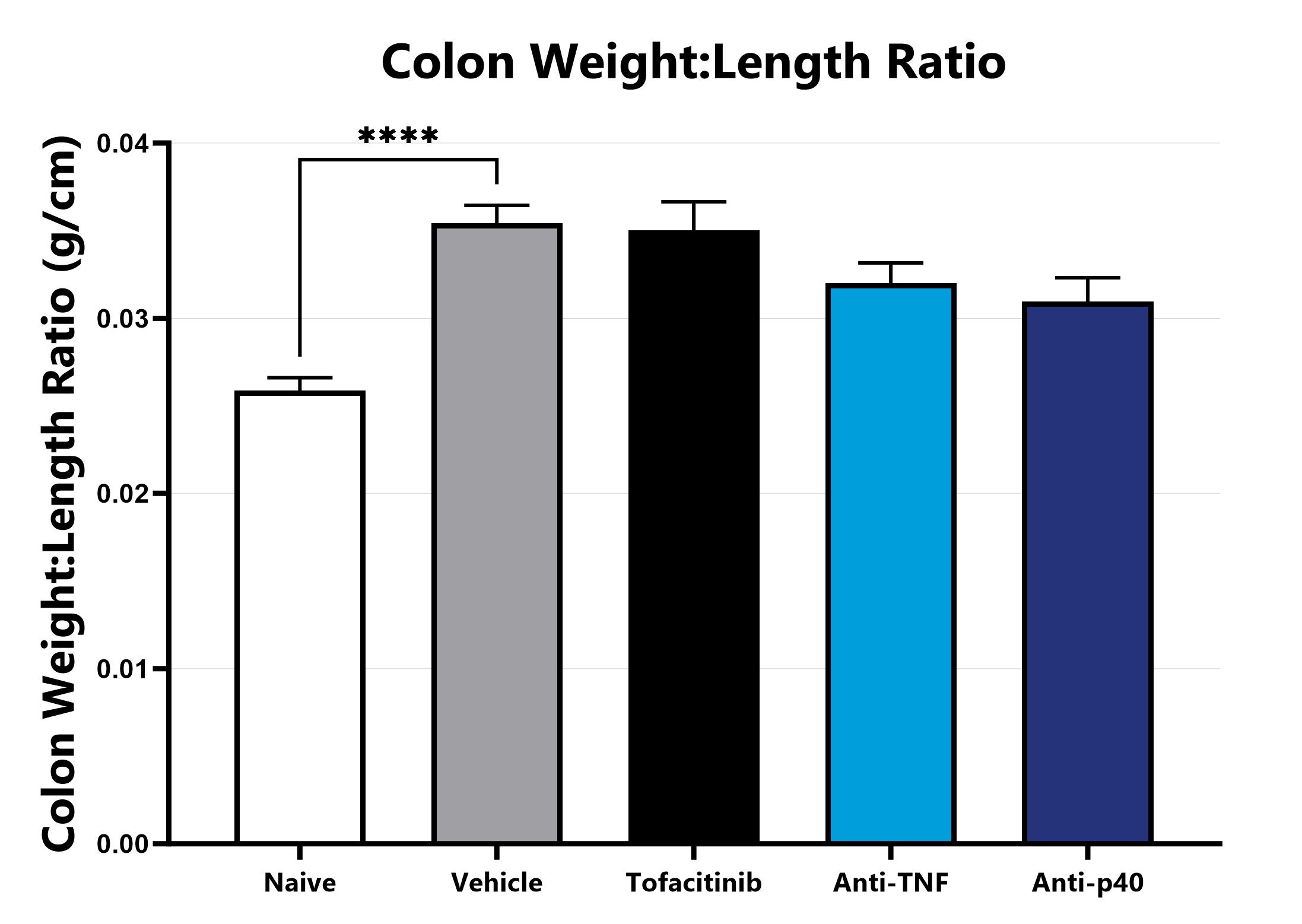
Following euthanasia, the colon is excised, measured, rinsed, and weighed; the colon weight:length ratio is calculated. (****p<0.001 compared to the vehicle-control).
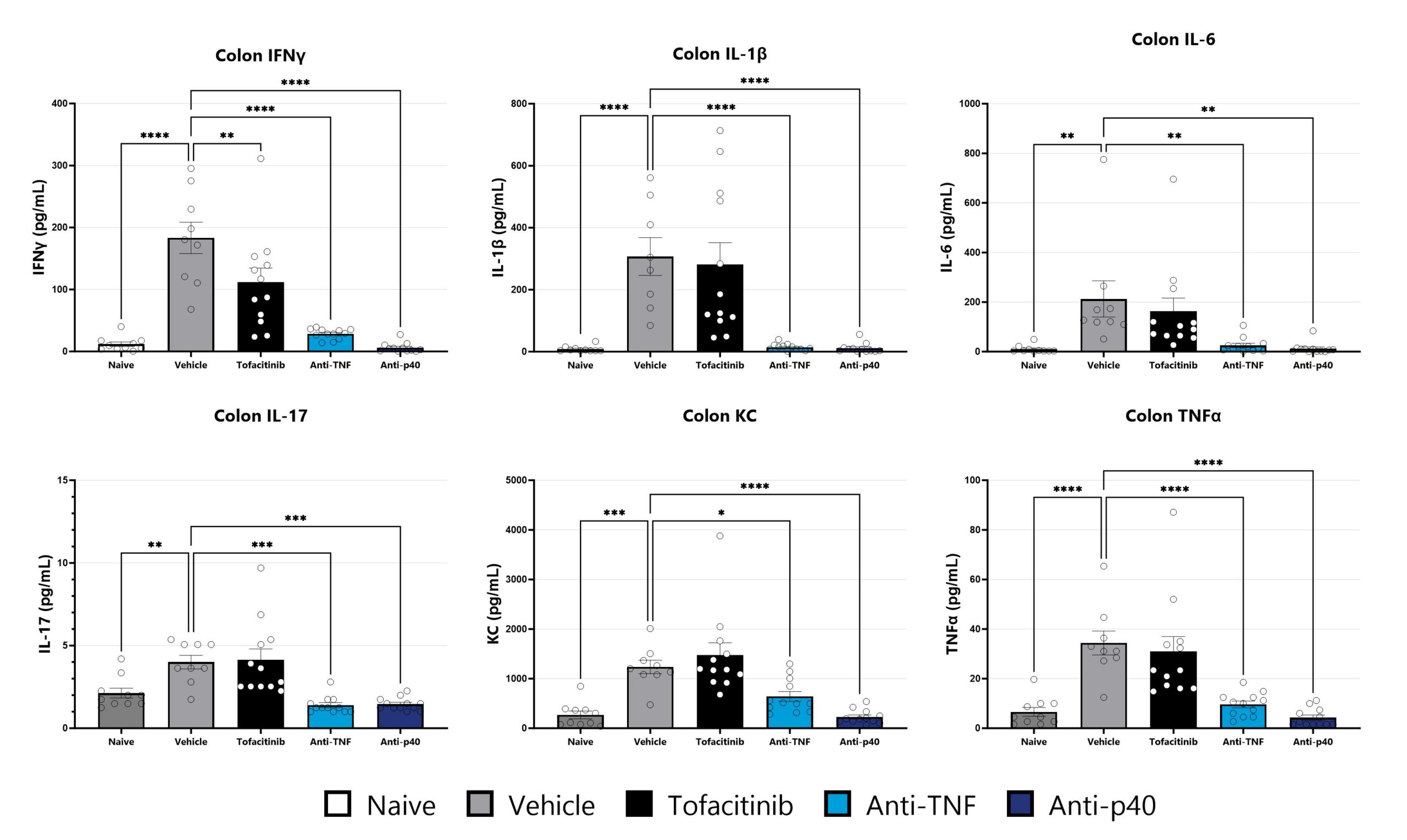
Colon samples from the CD40 mAb-induced colitis are homogenized and assessed via multiplex cytokine analysis for IFNγ, IL-1β, IL-6, IL-17, KC, and TNFα. Additional cytokines are available. (*p<0.05; **p<0.01; ***p<0.005; ****p<0.001 compared to the vehicle-control).

Close

The naïve T cell transfer model of colitis is the most widely used model to assess IBD immunopathology. The model is frequently used in drug development, particularly for those drugs with a T cell-dependent mechanism of action; T cell-dependent models of IBD are considered by many to be the most relevant to human disease. The model presents with inflammatory cell infiltration within the lamina propria and mucosal epithelium, colonic gland loss, and epithelial hyperplasia, with erosion observed more rarely. Colitis is induced via adoptive transfer of naïve TH cells isolated from C57Bl/6 donors into RAG2-/- recipients. T cell engraftment is assessed via flow cytometry two weeks after adoptive transfer, and a reduction in weight gain or overt weight loss is observed approximately three weeks after transfer. Mortality is typically associated with disease induction and colitis remains robust through the experimental duration. Test article responses can be compared to the effects of either anti-TNF or anti-p40 mAb.
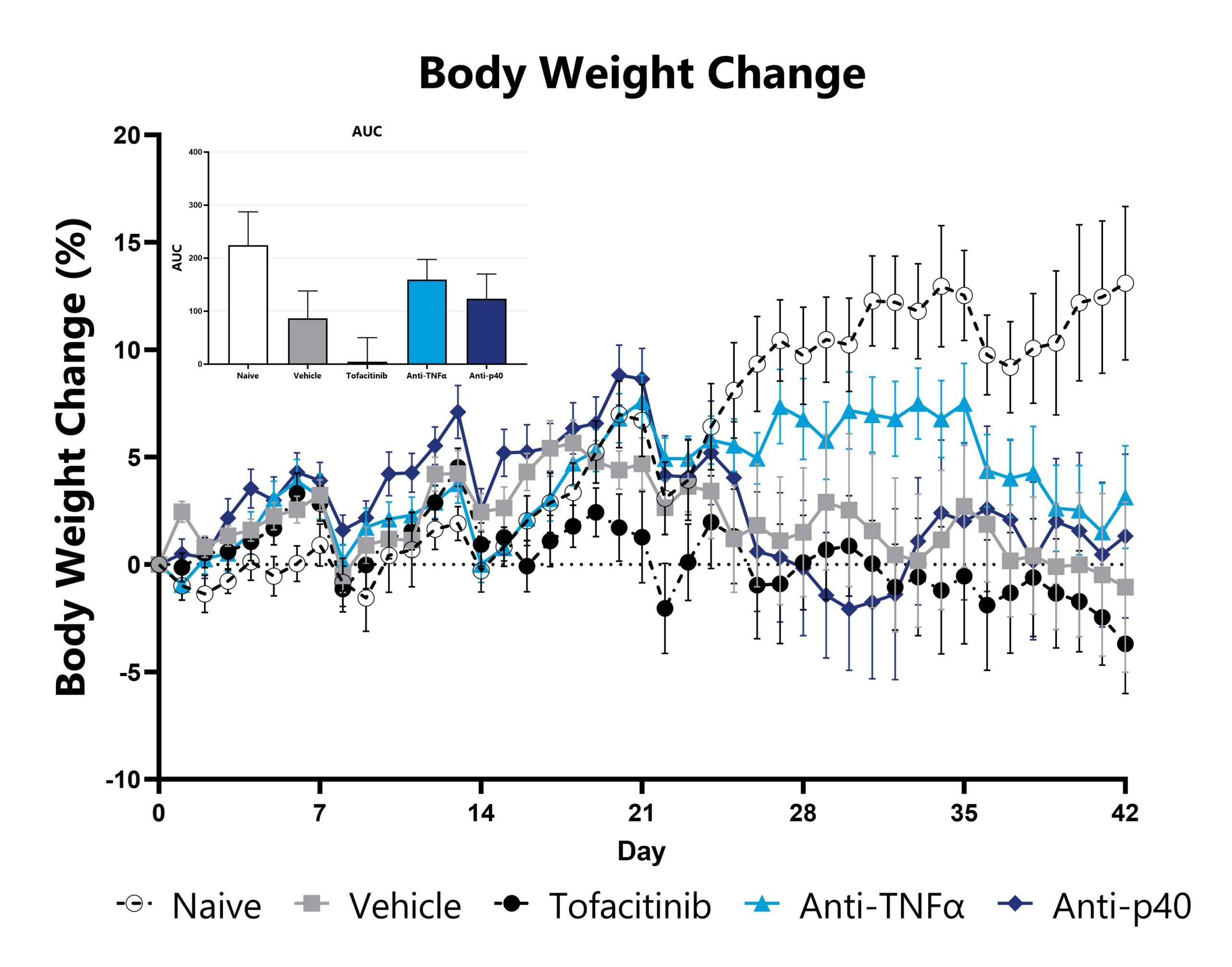
Naïve T cell transfer-induced animals are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset.
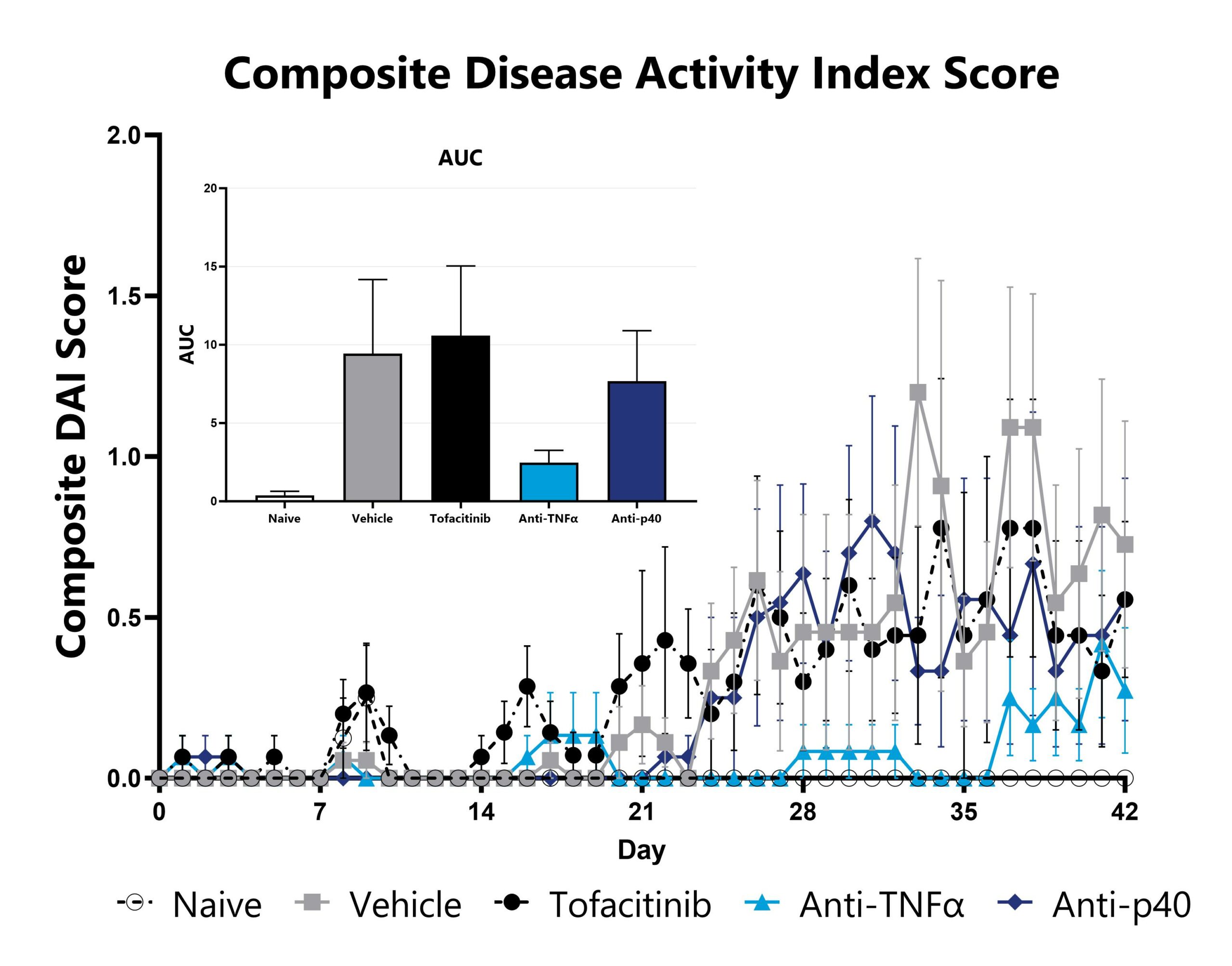
Naïve T cell transfer-induced animals are assessed daily for weight loss, diarrhea, and blood in stool phenotypes. Each phenotype is scored and used to calculate the composite Disease Activity Index score. The AUC is calculated to compare treatment arms and is shown in the inset.
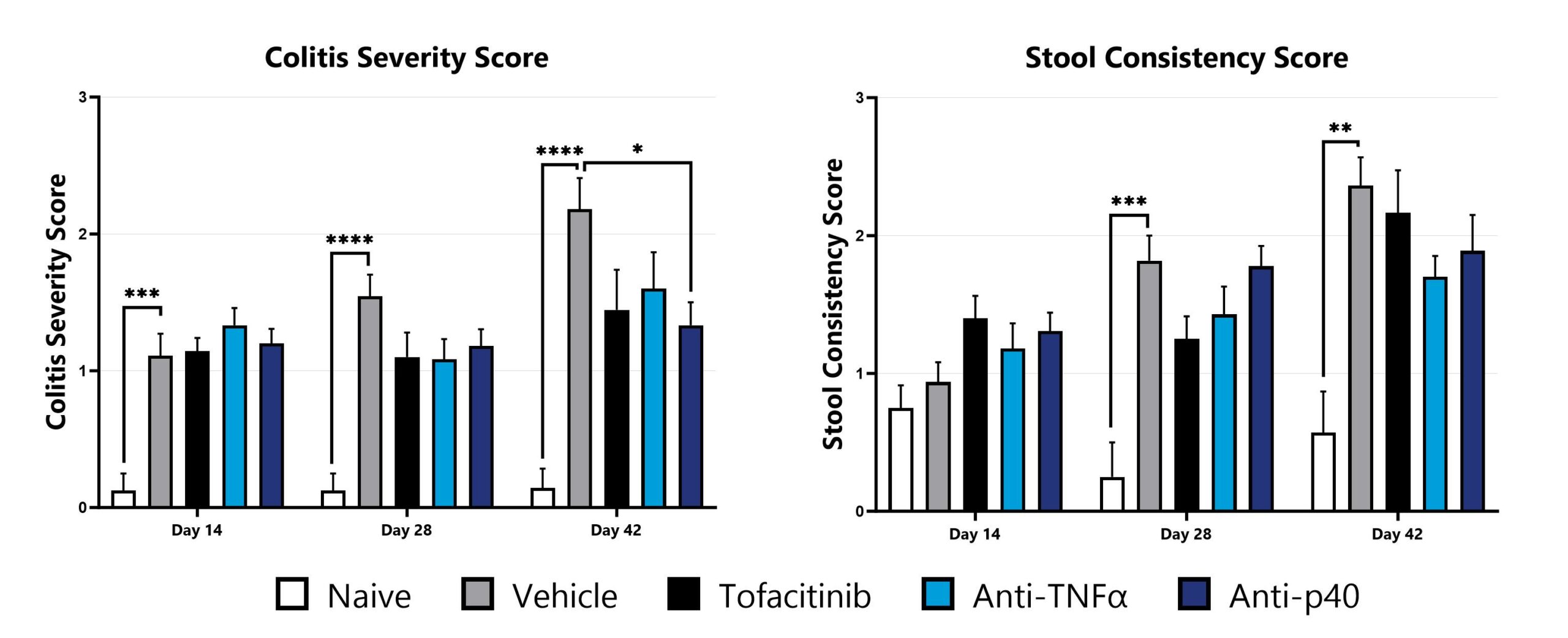
Naïve T cell transfer-induced animals are assessed for colon inflammation and ulceration by video endoscopy and are scored for colitis severity (left) and stool consistency (right). (*p<0.05; **p<0.01; ***p<0.005; ****p<0.001 compared to the vehicle-control).
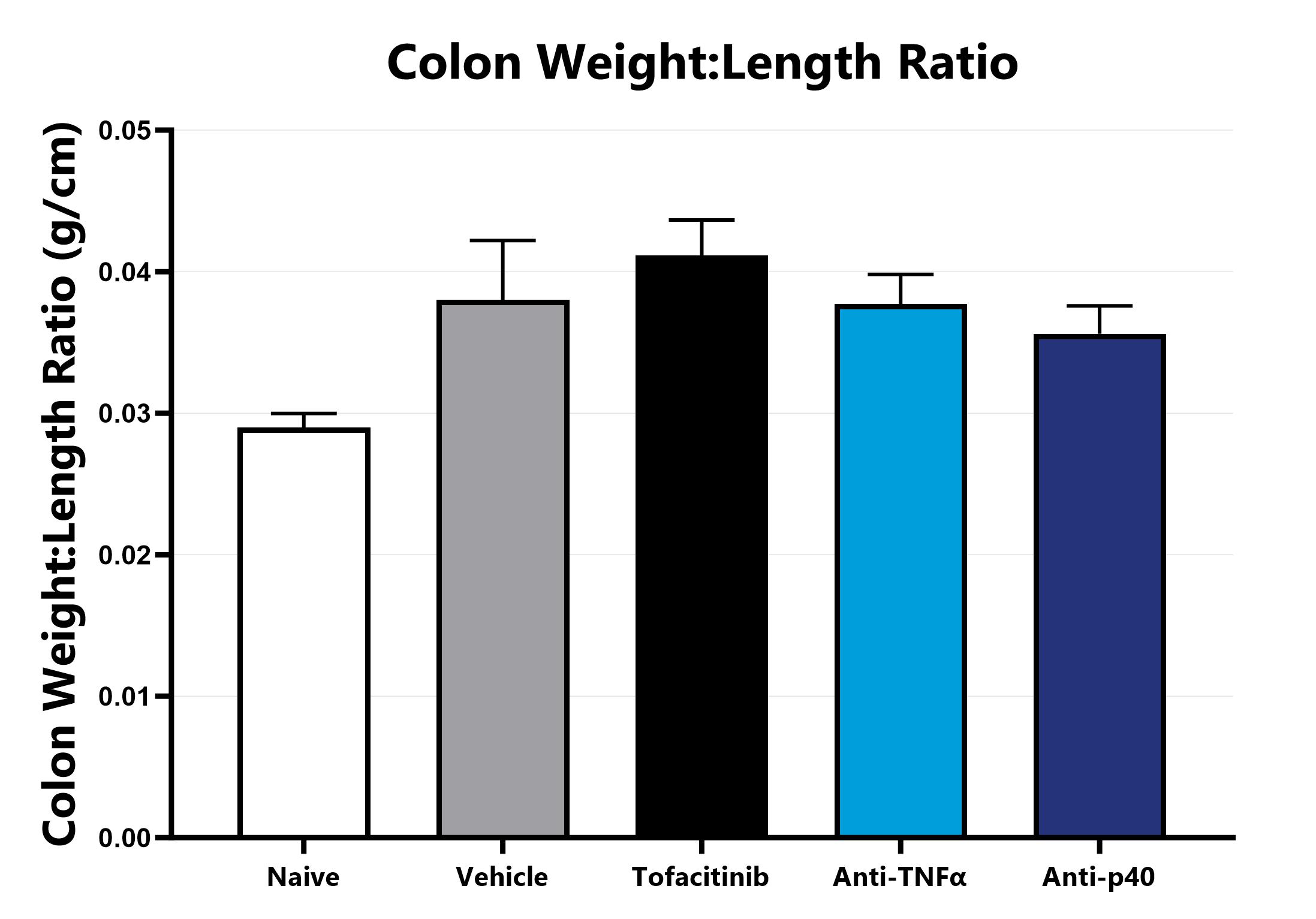
Following euthanasia, the colon is excised, measured, rinsed, and weighed; the colon weight:length ratio is calculated.
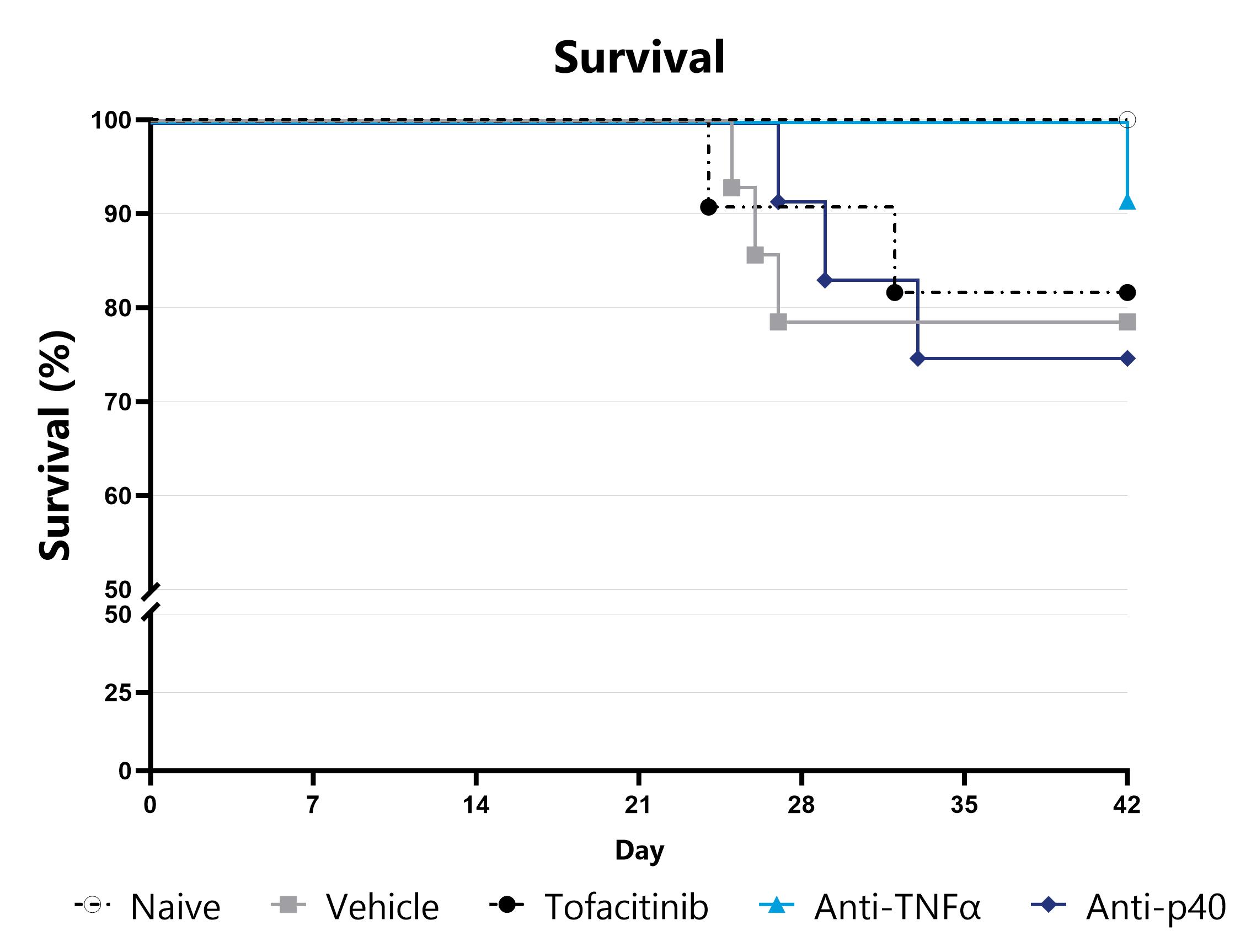
Animals are assessed for survival and moribundity on a daily basis and survival statistics (Kaplan-Meier) are determined.
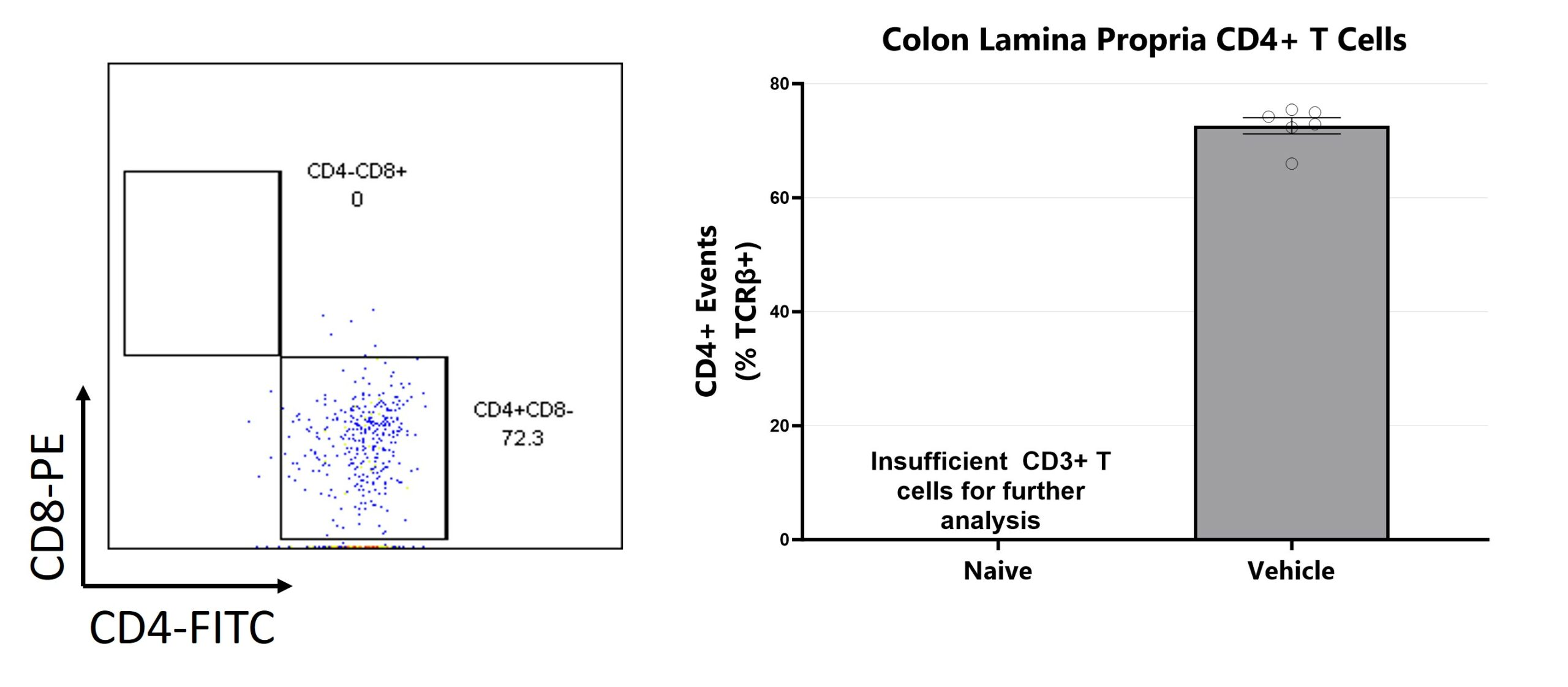
Colon samples are digested for lymphocyte enrichment, stained, and are assessed via Flow Cytometry for TH cells (CD4+CD8- among viable CD3+CD45+ cells).
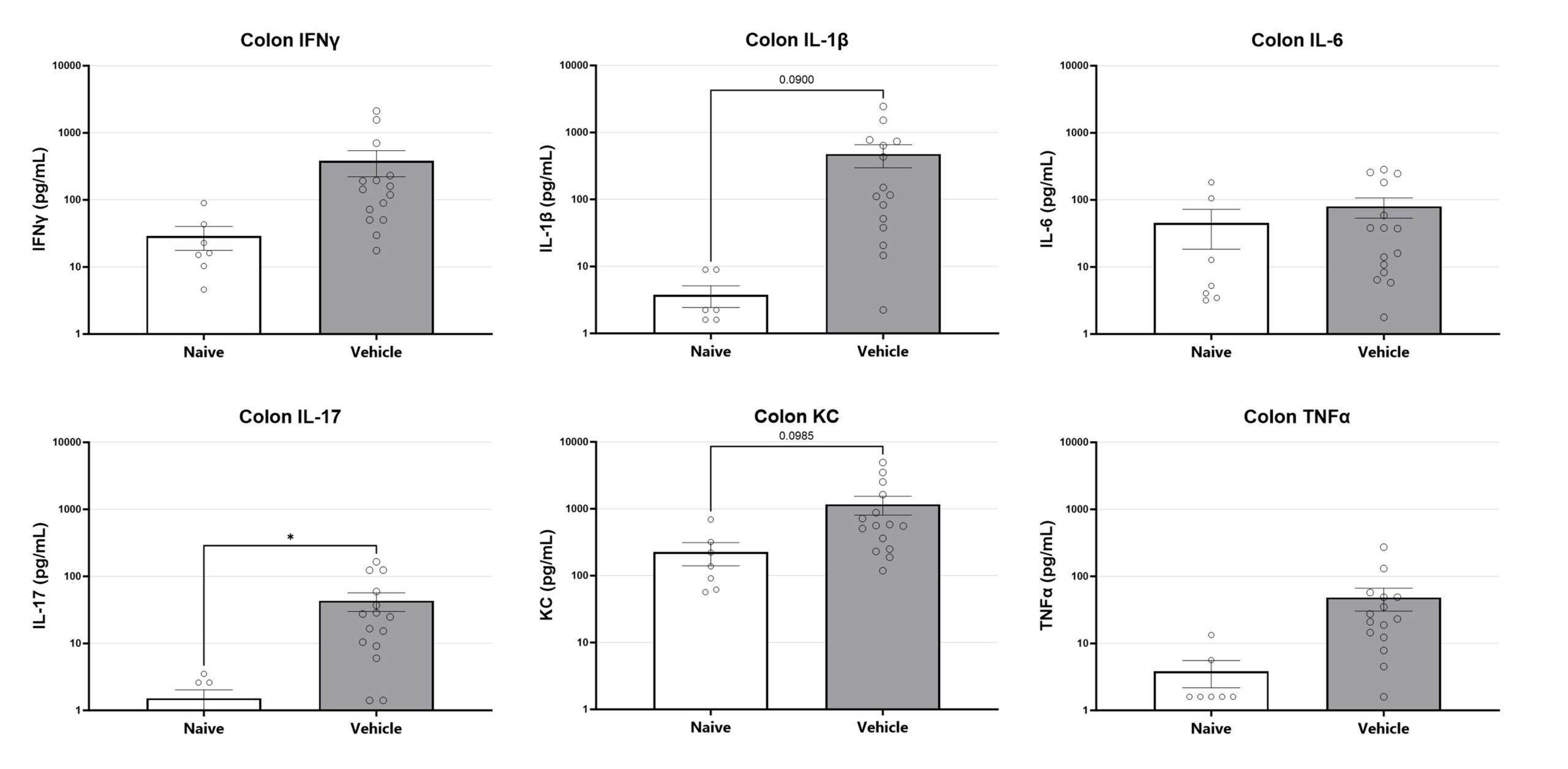
Colon samples are homogenized and assessed via multiplex cytokine analysis for IFNγ, IL-1β, IL-6, IL-17, KC, and TNFα. Additional cytokines are available. (*p<0.05)

Close

The IL-10 knockout mouse model is among the most utilized models for studying IBD and colitis. Colitis severity and onset can be variable in conventional animals and the colitis phenotype is extremely microbiome-dependent. Administration of a fecal microbial transplant (FMT) to germ-free IL-10 knockout animals (GF IL-10 KO) induces colitis characterized by inflammatory cell infiltration in the lamina propria and epithelial hyperplasia. Elevated fecal LCN2 and calprotectin are also observed. Test article responses can be compared to the effects of Anti-IL-12-p40 mAb.
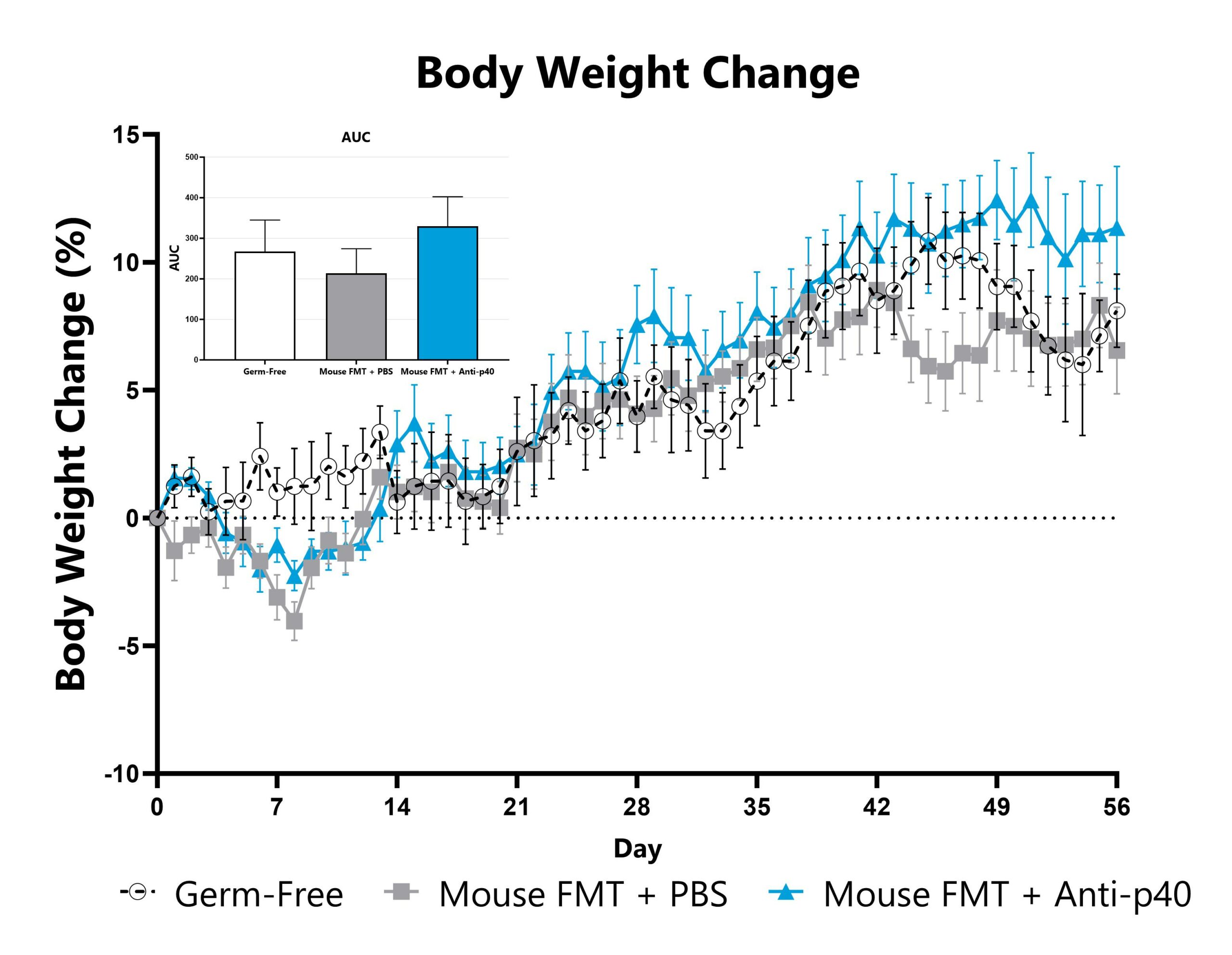
GF IL-10 knockout mice administered a mouse FMT are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset.
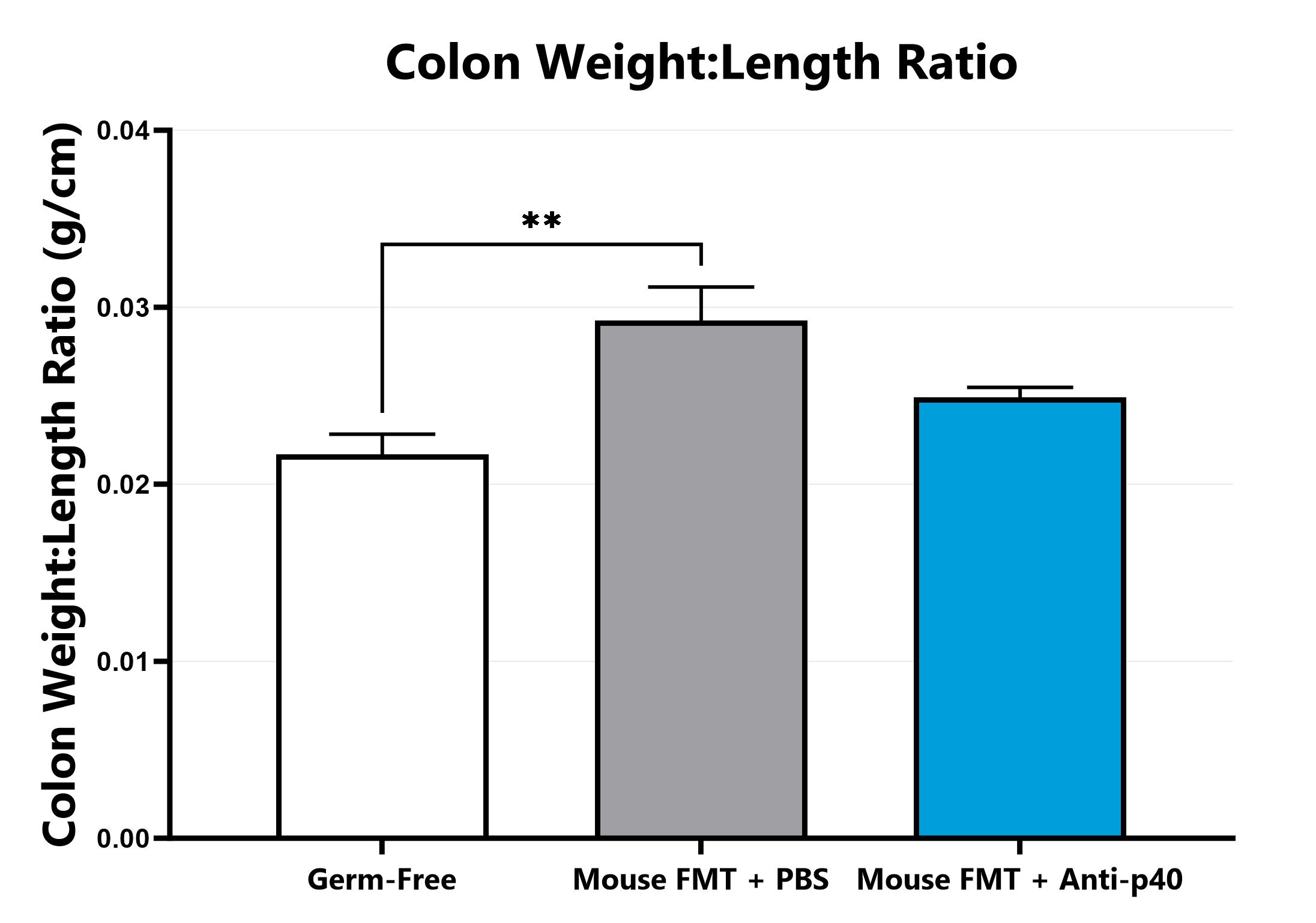
Following euthanasia, the colon is excised, measured, rinsed, and weighed; the colon weight:length ratio is calculated. (*p<0.05 compared to the vehicle-control).
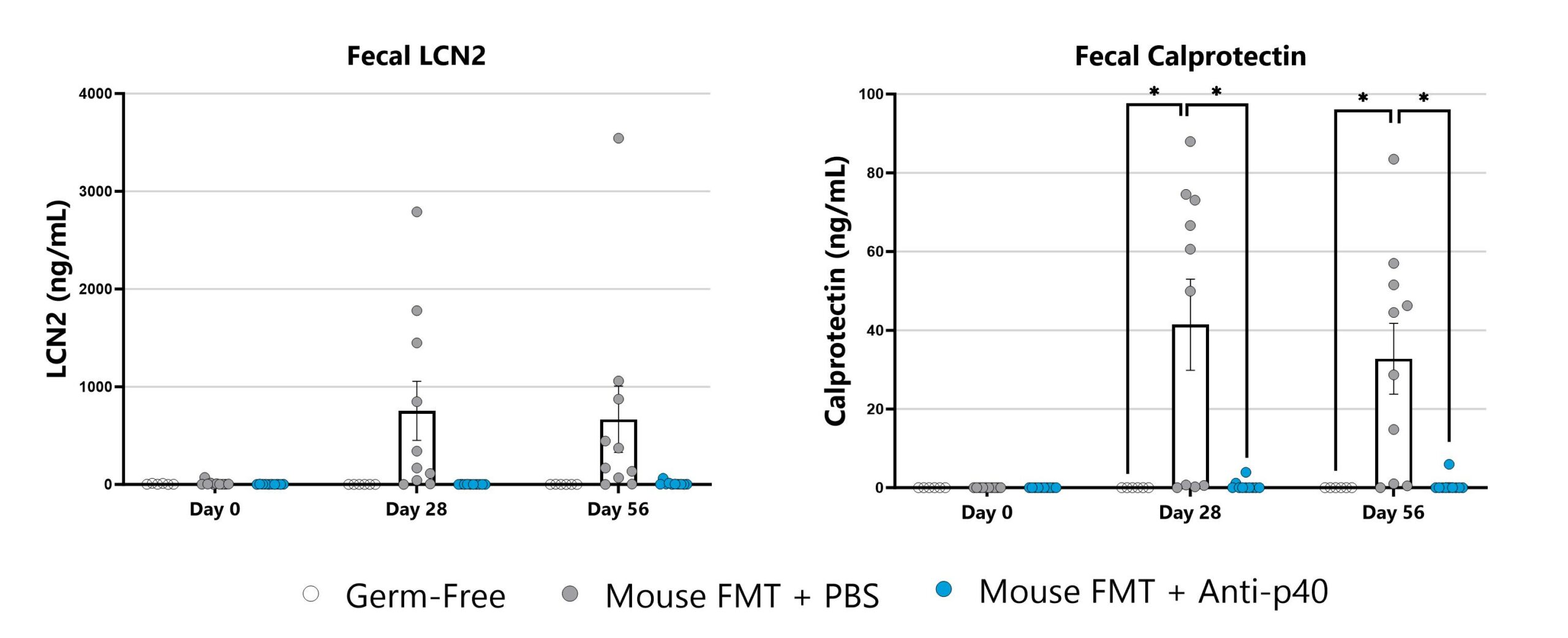
Lipocalin (left) and calprotectin (right) are measured in fecal samples by commercially available ELISA. (*p<0.05 compared to the vehicle-control).
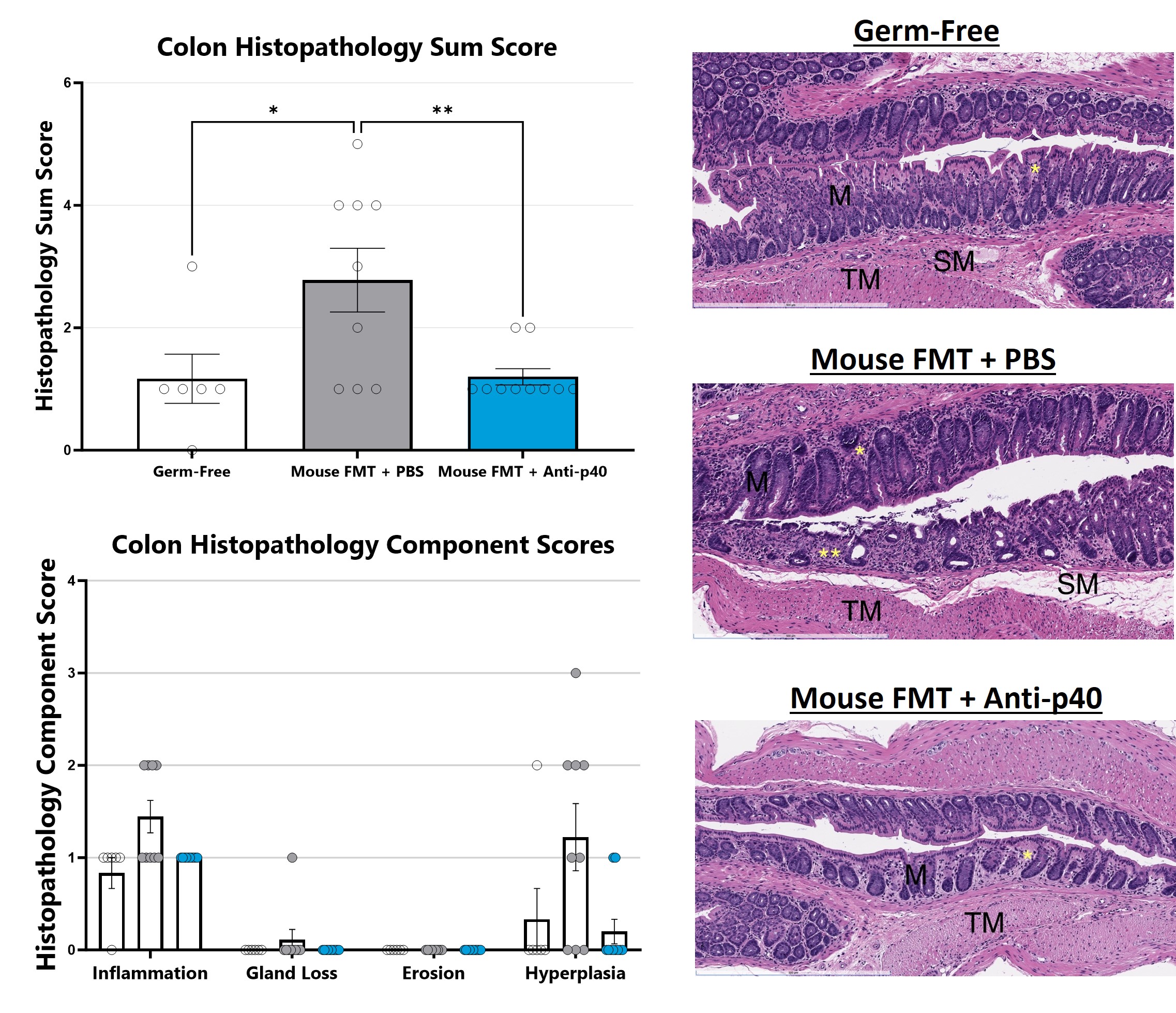
H&E stained mouse colon slides are evaluated with light microscopy. Features of inflammation, necrosis/gland loss, erosions, submucosal edema, and epithelial hyperplasia are given an individual score and scores are added together to obtain a sum score. Naïve is shown in upper right, vehicle is shown in middle right, and reference compound is shown in lower right. (*p<0.05; **p<0.001 as compared to vehicle control). Histopathology performed by Dallas Tissue Research.
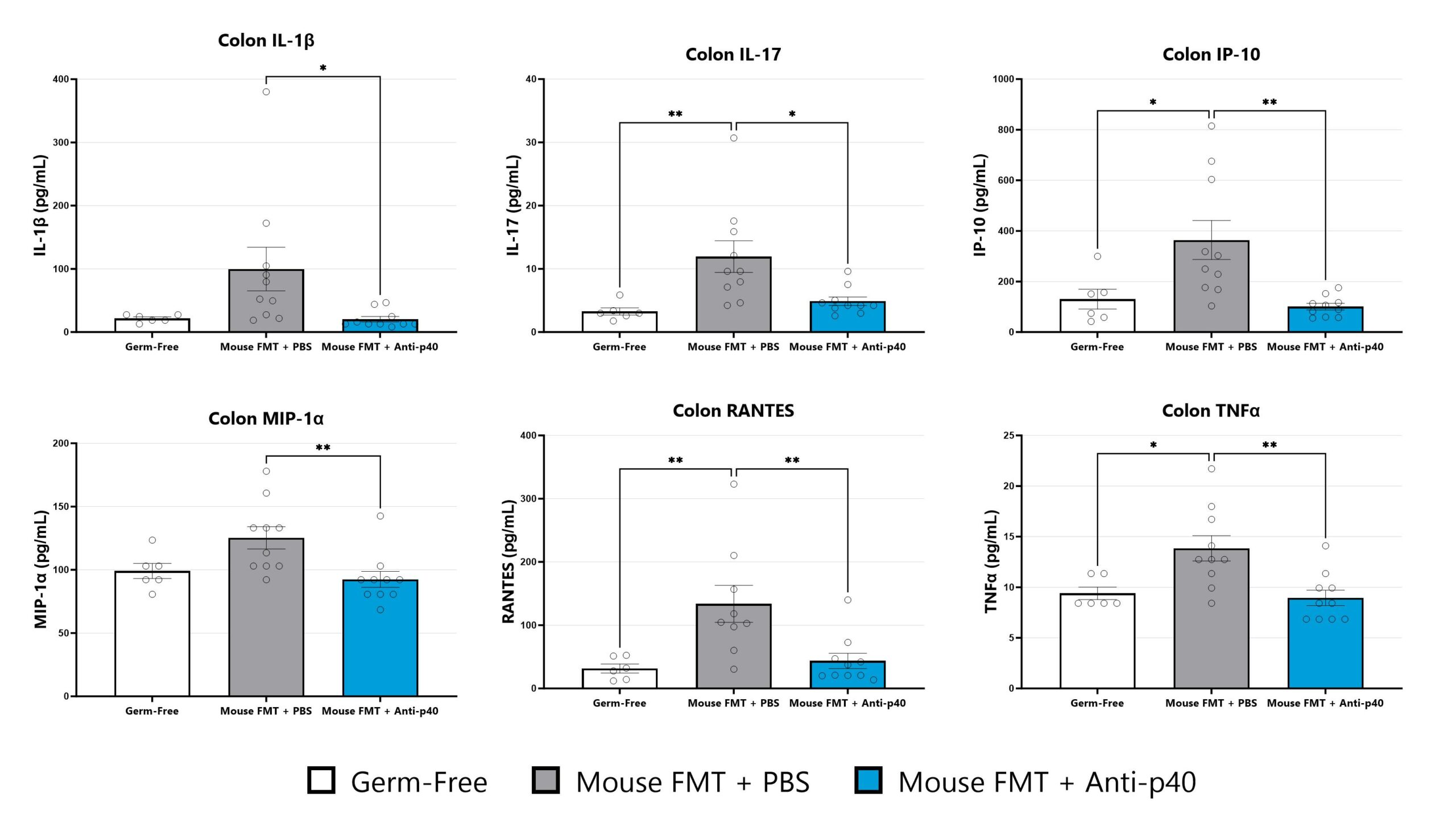
Colon samples are homogenized and assessed via multiplex cytokine analysis for IL-1β, IL-17, IP-10, MIP-1α, RANTES, and TNFα. Additional cytokines are available. (*p<0.05; **p<0.01 as compared to vehicle control).

Close

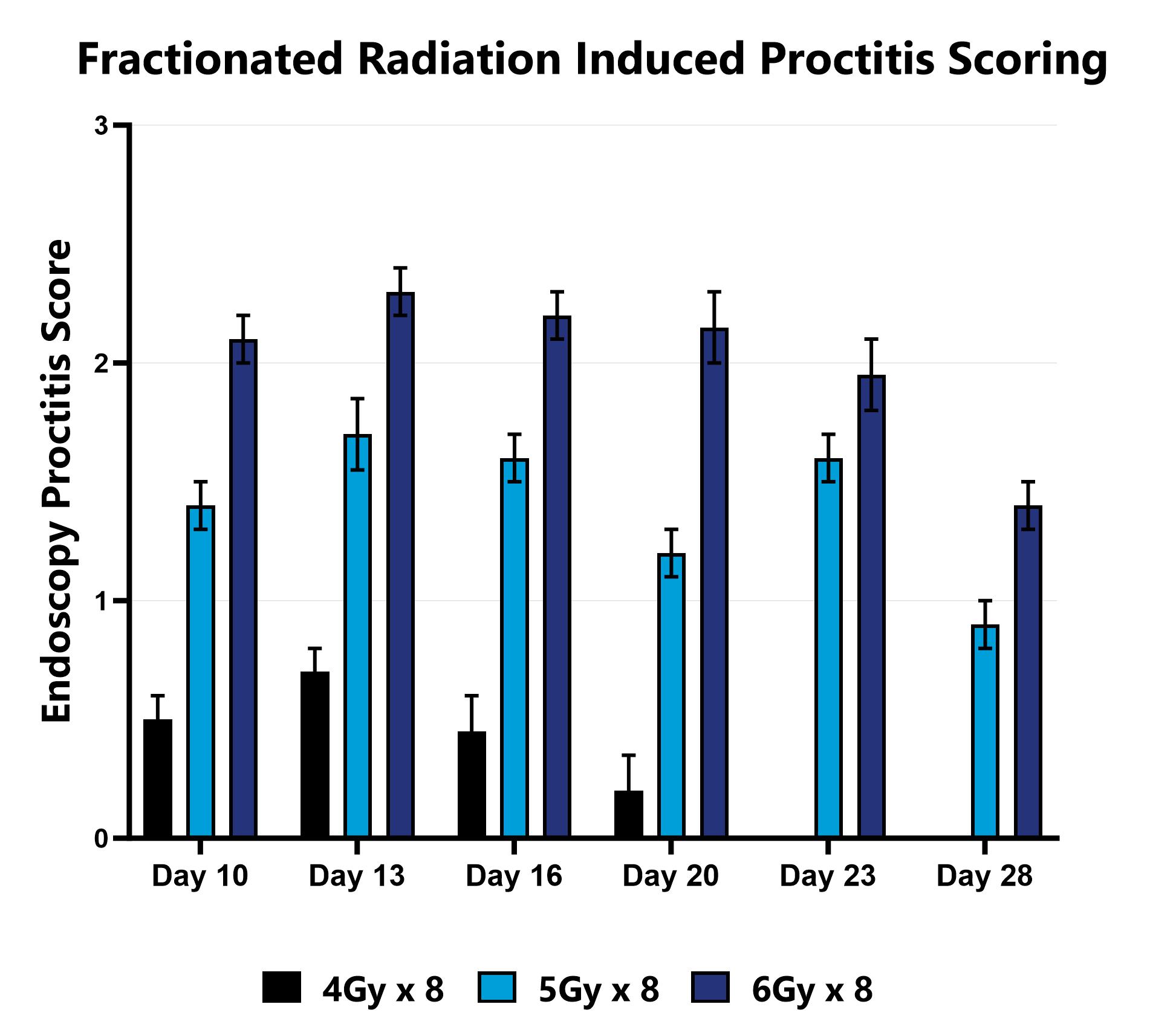
The direct effect of ionizing radiation during radiation therapy damages the DNA, lipids, and proteins of cells (both cancerous and non-cancerous) in the GI tract. In the rodent models, proctitis is induced with either a single bolus of acute radiation, or 8 fractionated, smaller doses, directed at approximately the most distal 2 cm of the colon (a lead shield protects the remainder of the animal’s body). Animals are monitored daily and evaluated for overall health and survival in addition to diarrhea and bloody stool incidence. A major readout in the model is proctitis severity determined via longitudinal video endoscopic assessments over the course of the study. Peak disease differs based on the method of induction, with disease persisting through the final evaluation which typically includes histopathology.
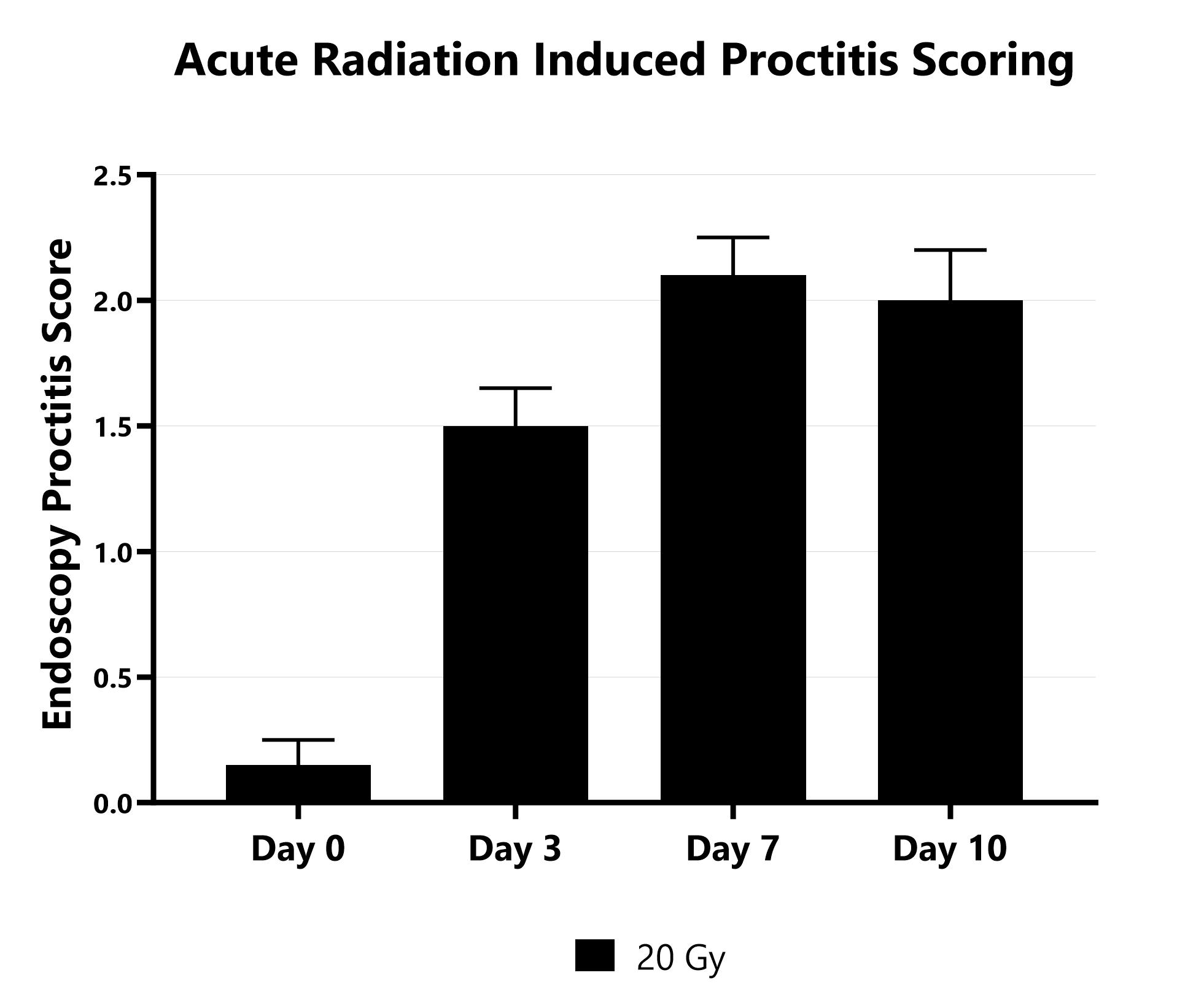
Proctitis severity is assessed longitudinally using video endoscopy at multiple timepoints during an acute radiation-induced proctitis study.
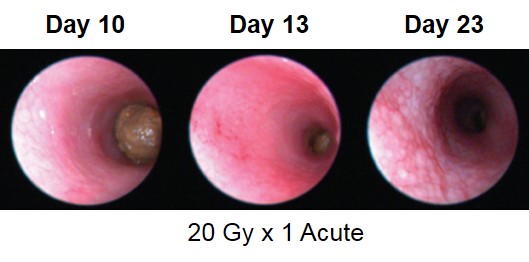
Proctitis severity is assessed longitudinally using video endoscopy at multiple timepoints during an acute radiation-induced proctitis study.
Proctitis severity is assessed longitudinally using video endoscopy at multiple timepoints during a fractionated radiation-induced proctitis study.
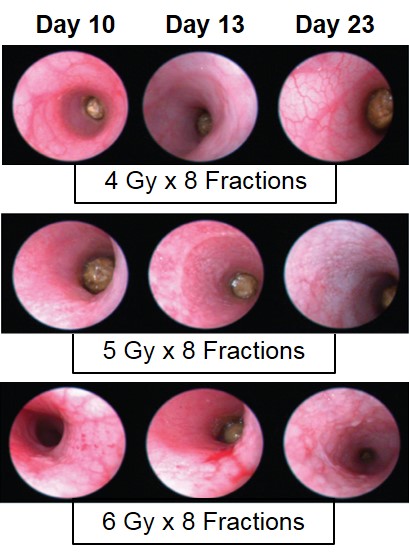
Proctitis severity is assessed longitudinally using video endoscopy at multiple timepoints during a fractionated radiation-induced proctitis study.
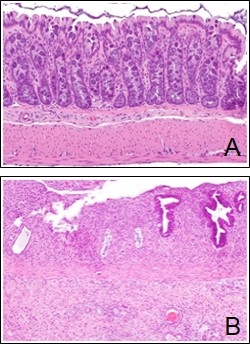
Following fractionated radiation treatment, rectal tissue is collected. (A) Photomicrograph showing histological appearance of normal rectal mucosa. (B) Photomicrograph showing histological appearance of inflamed rectal mucosa.

Close
Study Models

The H2b -> H2d major histocompatibility complex (MHC) mismatched model is the most commonly studied GVHD model and is often used in drug discovery and development. Acute GVHD involves the cytotoxic effects of donor T cells on recipient tissues, the activation of APCs and an inflammatory cascade. Recipient mice (H2d) are pre-conditioned with a lethal dose of total body irradiation prior to the adoptive transfer of T-cell depleted bone marrow supplemented with splenocytes harvested from an MHC-mismatched donor mouse (H2b). Following transplant, the mice rapidly lose weight due to the conditioning regimen and then partially recover by Day 14, after which they develop a progressively worsening disease characterized by weight loss and increasing GVHD score.
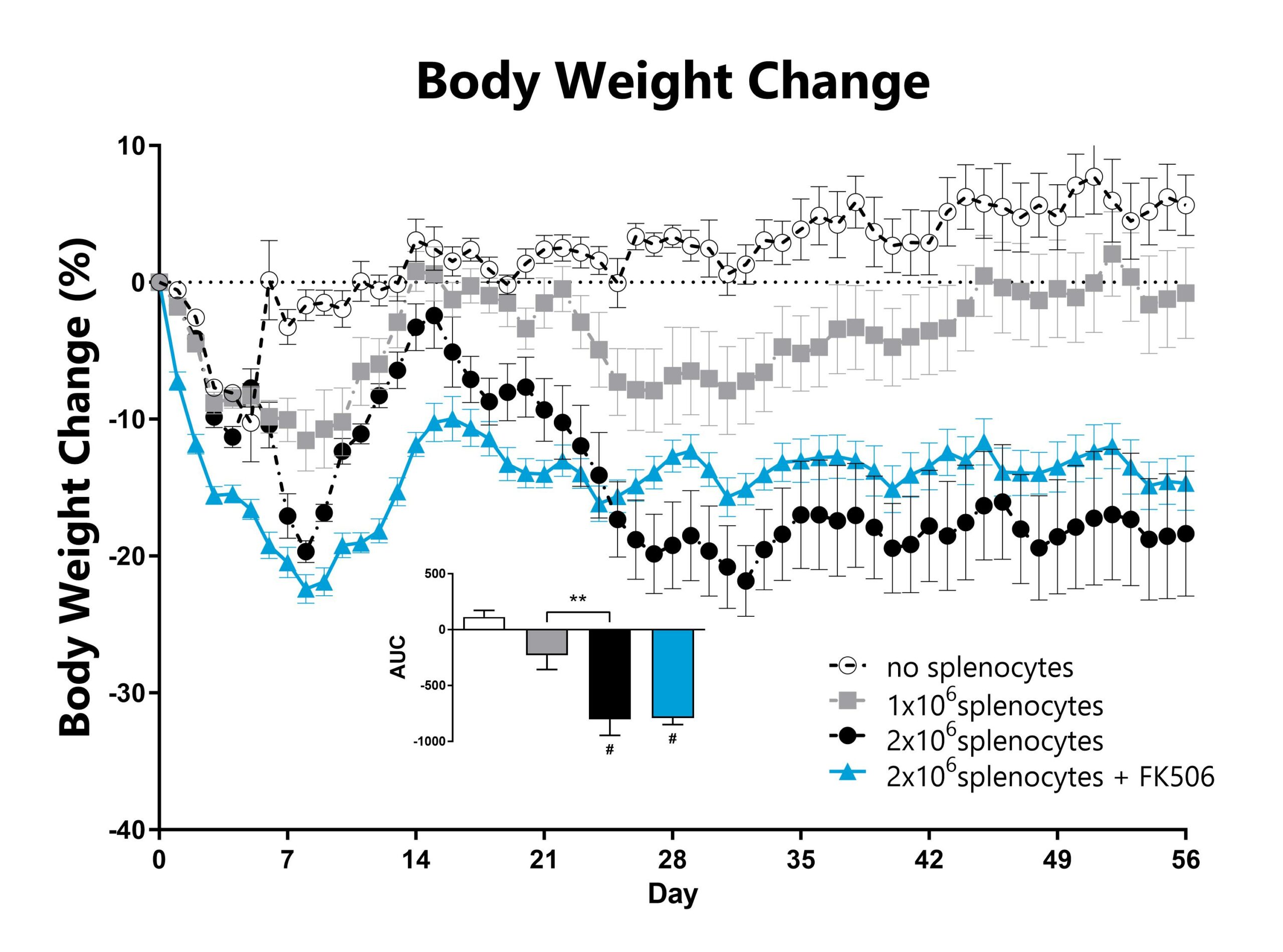
Recipient mice are pre-conditioned and transplanted with donor-derived CD3-depleted bone marrow cells supplemented with splenocytes on Day 0. Animals are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment groups and is shown in the inset (**: p<0.01; #: p<0.05 compared to the ‘no splenocytes’ control).
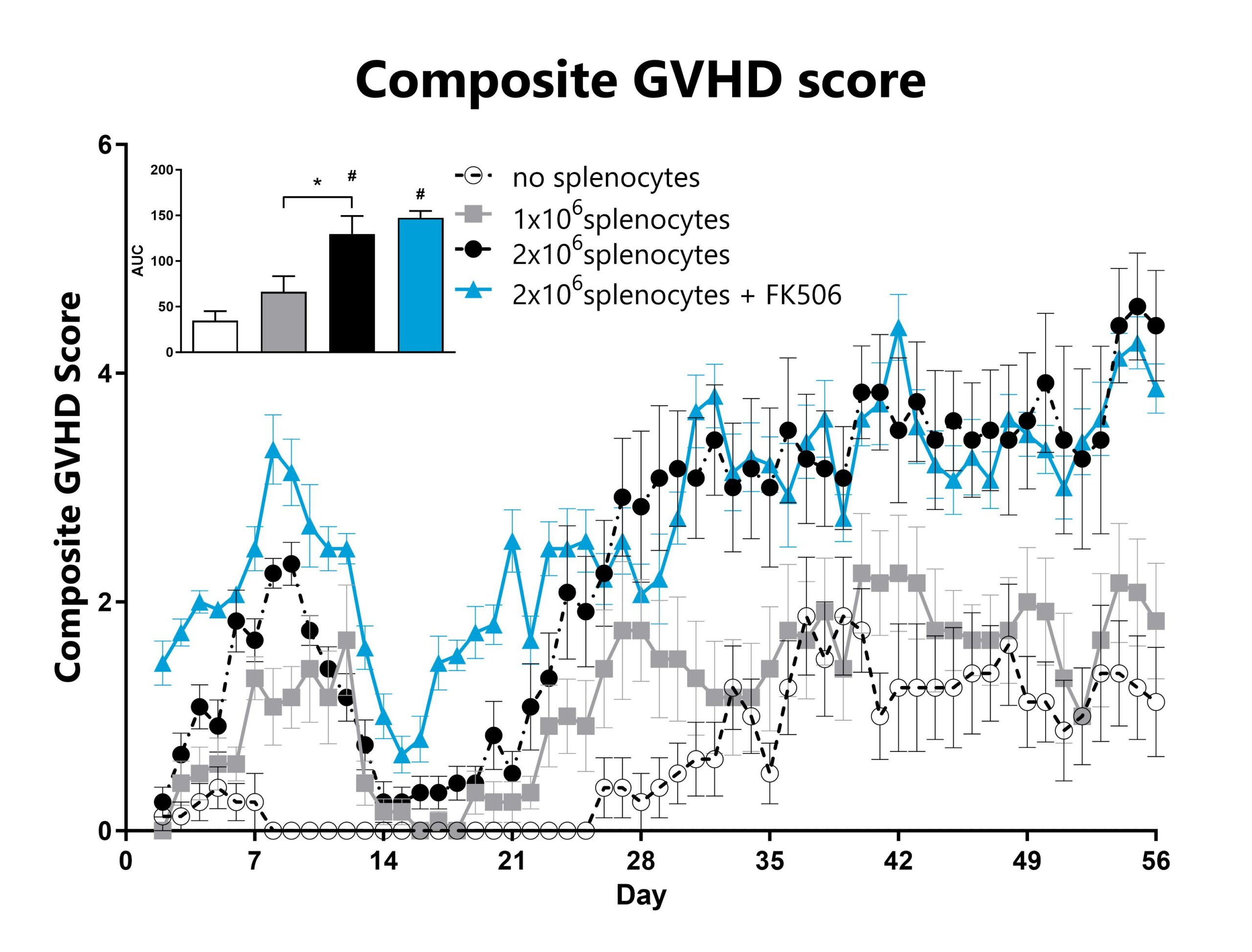
GVHD mice are assessed daily for weight loss, posture, activity, fur texture, and skin integrity phenotypes using our standard GVHD scoring scale. Each phenotype is scored and used to calculate the composite GVHD score. The AUC was calculated to compare treatment groups and is shown in the inset. (*: p<0.05; #: p<0.05 compared to ‘no splenocytes’ control)
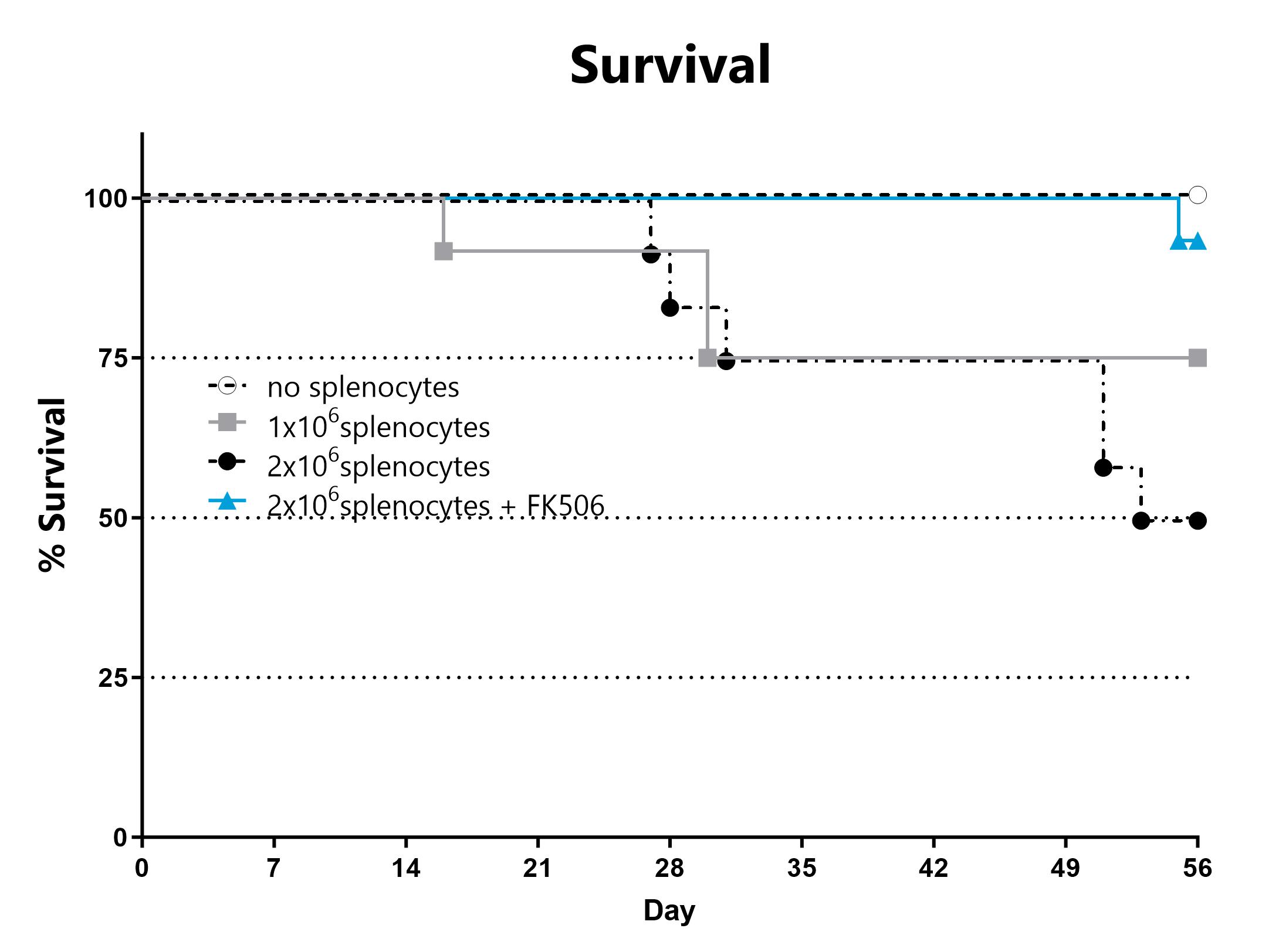
Animals are assessed for survival and moribundity on a daily basis.
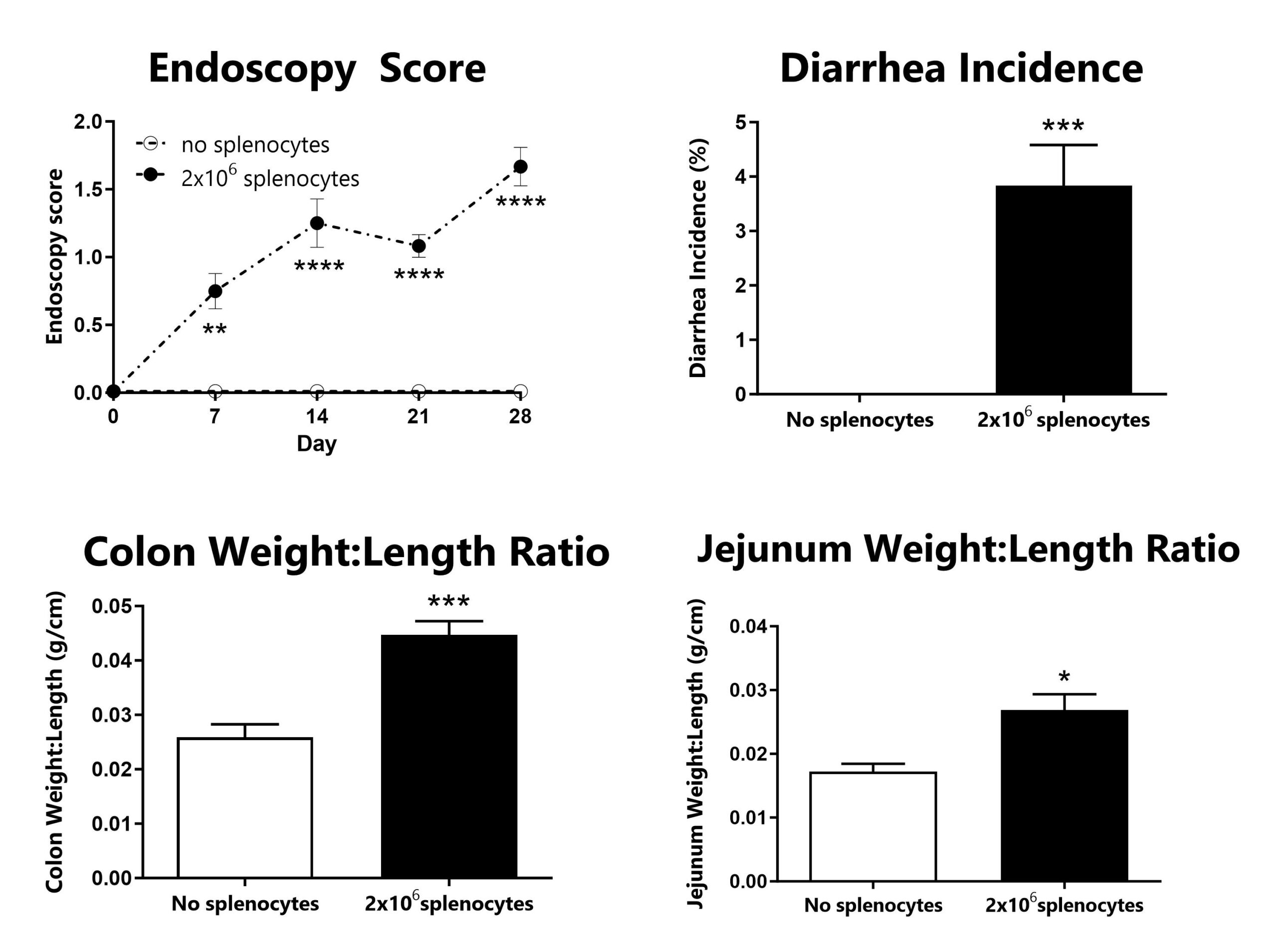
GVHD animals are assessed for colon inflammation by video endoscopy and scored for disease severity (top left). Diarrhea is monitored daily and used to calculate incidence of diarrhea (top right). Following euthanasia, the colon and jejunum are excised, measured, rinsed, and weighed; the colon weight:length ratio (bottom left) and jejunum weight:length ratio (bottom right) are calculated. (*: p<0.05; **: p<0.01; ***: p<0.005; ****: p<0.001 compared to ‘no splenocytes’ control)
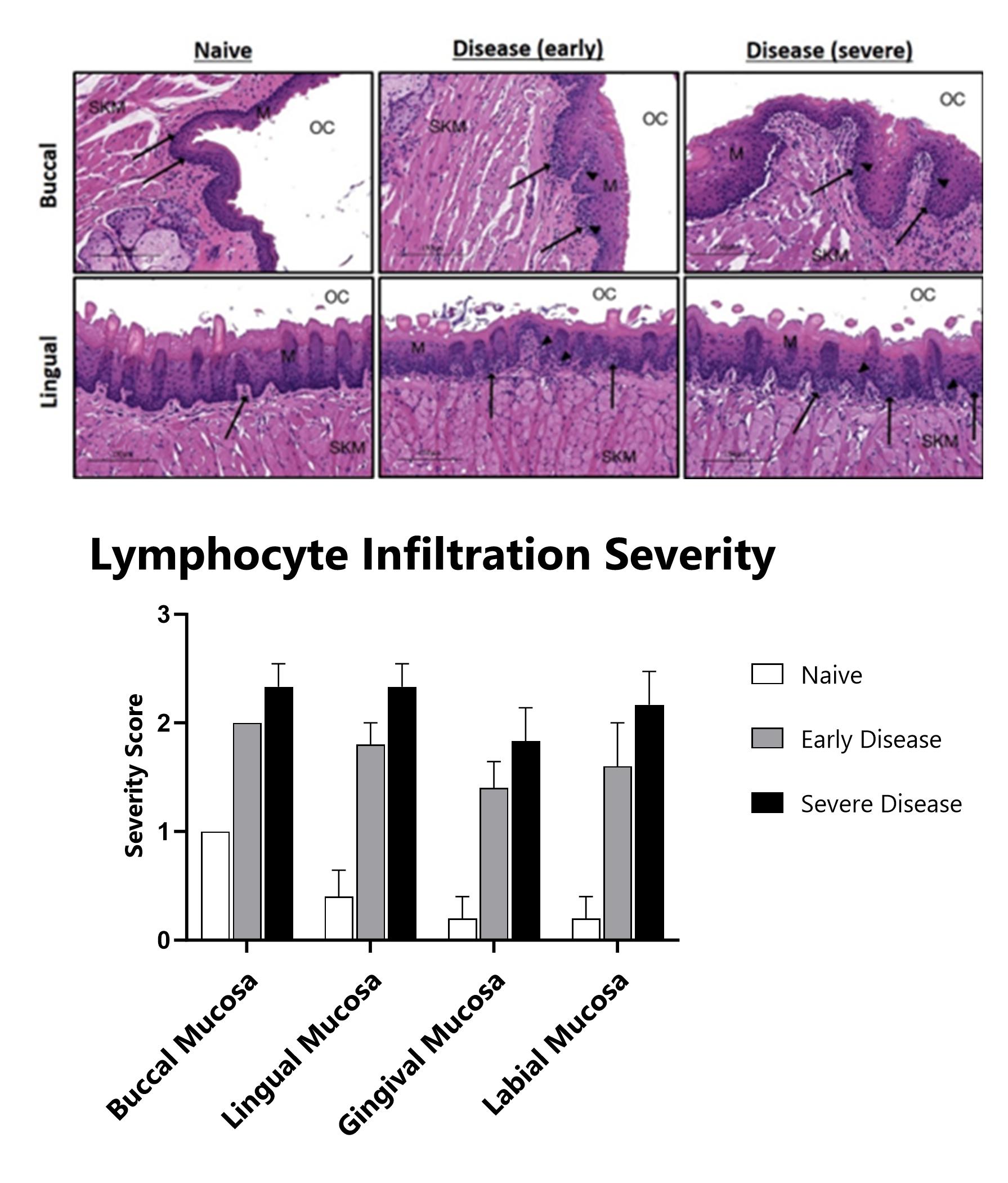
H&E stained oral mucosa lesions are scored for disease severity. Black arrows indicate lymphocytic infiltration. Black arrowheads indicate apoptotic bodies. OC – oral cavity. SKM – skeletal muscle. M – mucosa. Histopathology performed by Dallas Tissue Research.

Close

The Sclerodermatous Chronic GVHD model is a minor MHC mismatched model commonly used to study pathogenesis of fibrosis in cGVHD, and frequently used in drug discovery and development. Sclerodermatous Chronic GVHD model pathology involves immune cell mediated release of Th2 cytokines and fibrosis pathways. Recipient C57Bl/6 mice are pre-conditioned with a lethal dose of total body irradiation (TBI) prior to the adoptive transfer of bone marrow that is supplemented with splenocytes harvested from a minor MHC-mismatched donor mouse (LP/J). Following irradiation and transplant, the recipient mice show transient weight loss that typically recovers by Day 21 to 28. After this 3-4 week period, animals develop a progressively worsening GVHD that is characterized by weight loss, increasing GVHD score, and development of sclerodermatous skin lesions. Test article responses can be compared to the effects of Ruxolitinib.
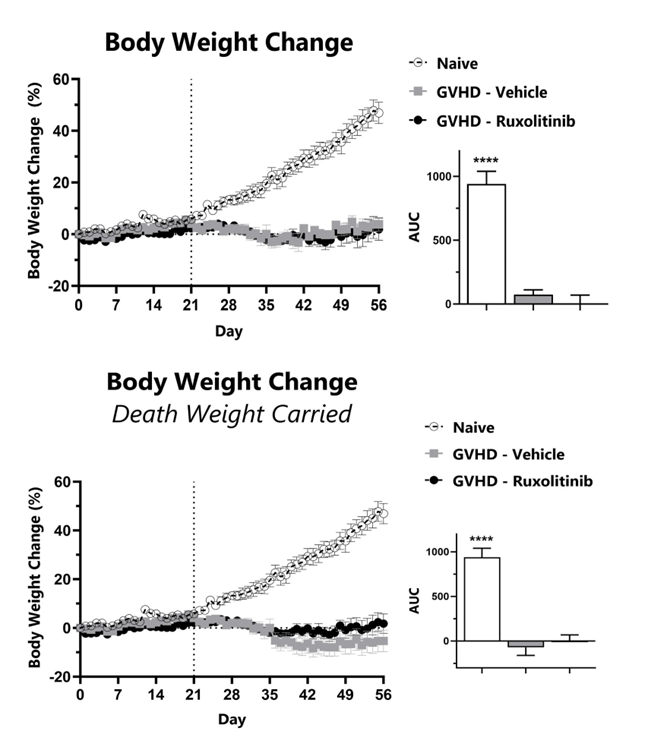
Recipient mice are pre-conditioned and transplanted with donor-derived bone marrow cells supplemented with splenocytes on Day 0. Animals are weighed daily, and body weight change as compared to Day 0 is calculated (top). To normalize for survivor bias, percent weight change with death weight carried forward is calculated (bottom). The AUC is calculated to compare treatment groups and is shown in the inset. (****: p<0.0001; compared to GVHD-Vehicle group)
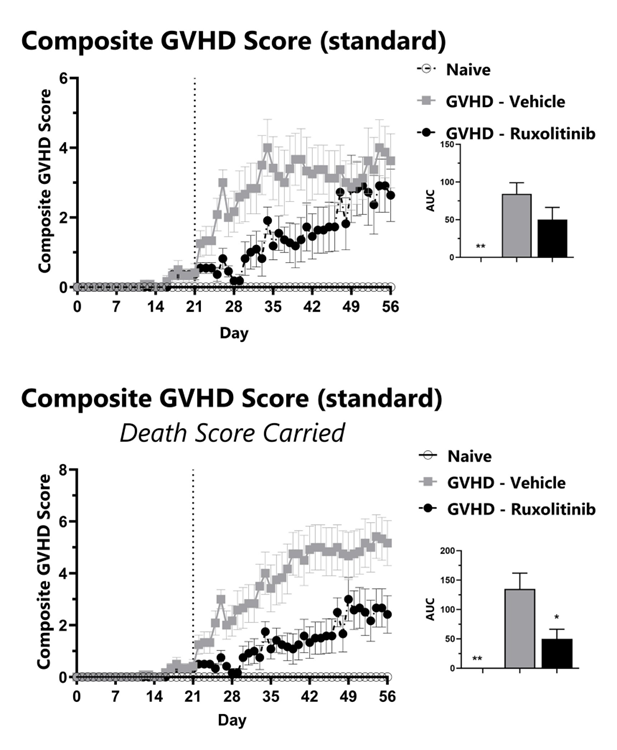
GVHD mice are assessed daily for weight loss, posture, activity, fur texture, and skin integrity phenotypes using our standard GVHD scoring scale. Each phenotype is scored and used to calculate the composite GVHD score (top). To normalize for survivor bias, GVHD score with death score carried forward is calculated (bottom). The AUC is calculated to compare treatment groups and is shown in the inset. (*: p<0.05; **: p<0.01 compared to GVHD-Vehicle group)
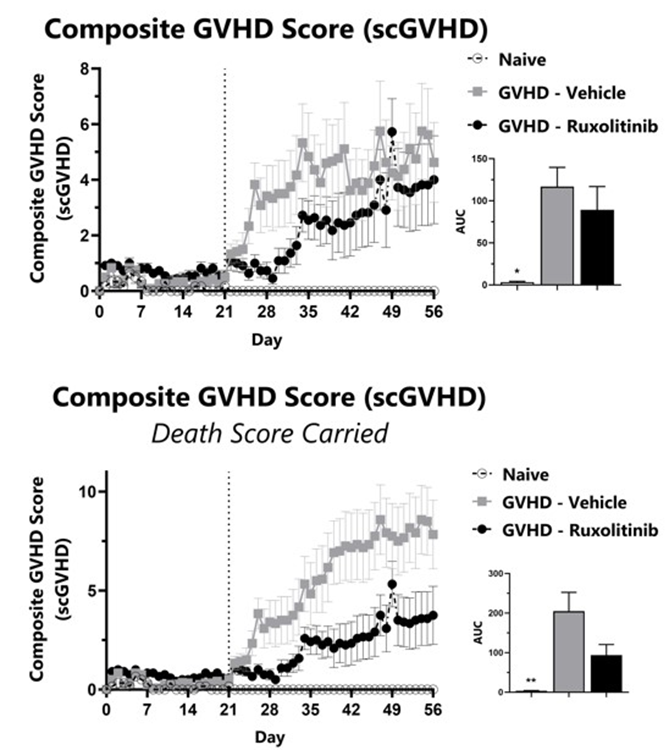
GVHD mice are assessed daily for weight loss, posture, activity, fur texture, and skin integrity phenotypes using our Sclerodermatous Chronic GVHD scale. Each phenotype is scored and used to calculate the composite Sclerodermatous Chronic GVHD score (top). To normalize for survivor bias, Sclerodermatous Chronic GVHD score with death score carried forward is calculated (bottom). The AUC is calculated to compare treatment groups and is shown in the inset. (*: p<0.05; **: p<0.01 compared to GVHD-Vehicle group)
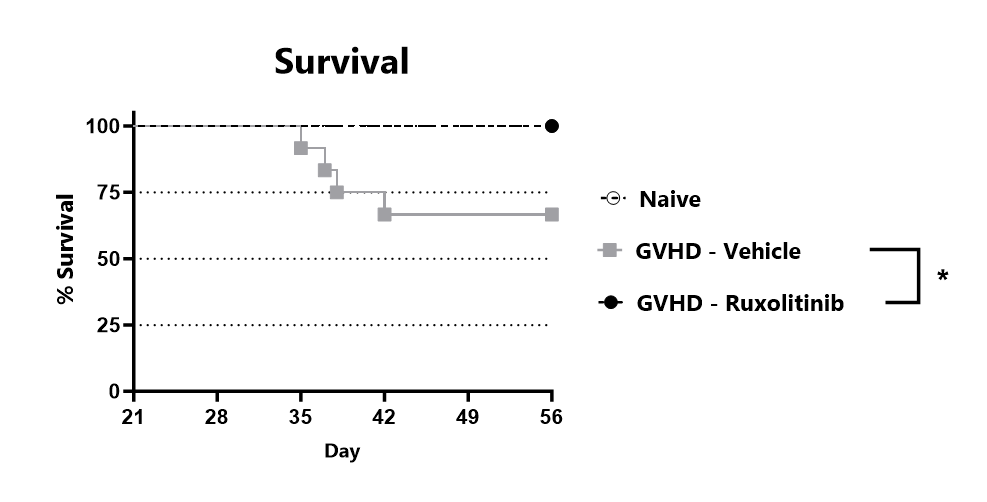
Animals are assessed for survival and moribundity on a daily basis. (*: p<0.05 compared to GVHD-Vehicle group)
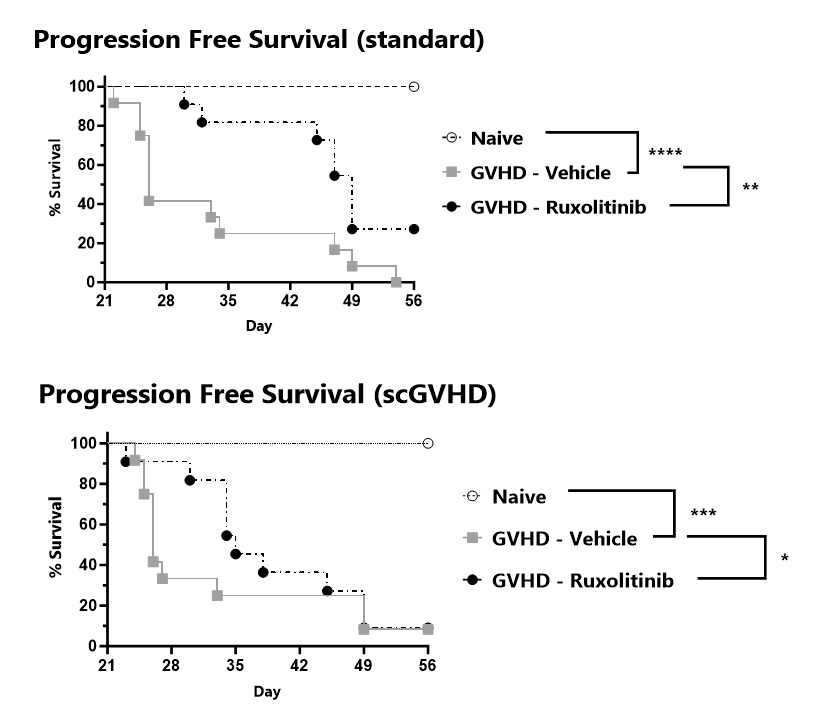
Progression free survival is tracked for the duration of the study and percent progression free survival is calculated for the standard GVHD scale (top) and the Sclerodermatous Chronic GVHD scale (bottom).

Close

The xGVHD model using NSG mice is the most permissive mouse model for the study of human T-cell engraftment and is often used in drug discovery and development. Transplantation of human peripheral blood mononuclear cells (huPBMCs) causes an Acute GVHD-like syndrome. Recipient severely immunocompromised (NSG) mice are injected with a pre-determined number of purified single-donor human PBMCs. Following transplant, the mice develop a progressively worsening disease characterized by weight loss and increasing GVHD score. Endpoint survival in untreated mice is determined by the number of cells injected.
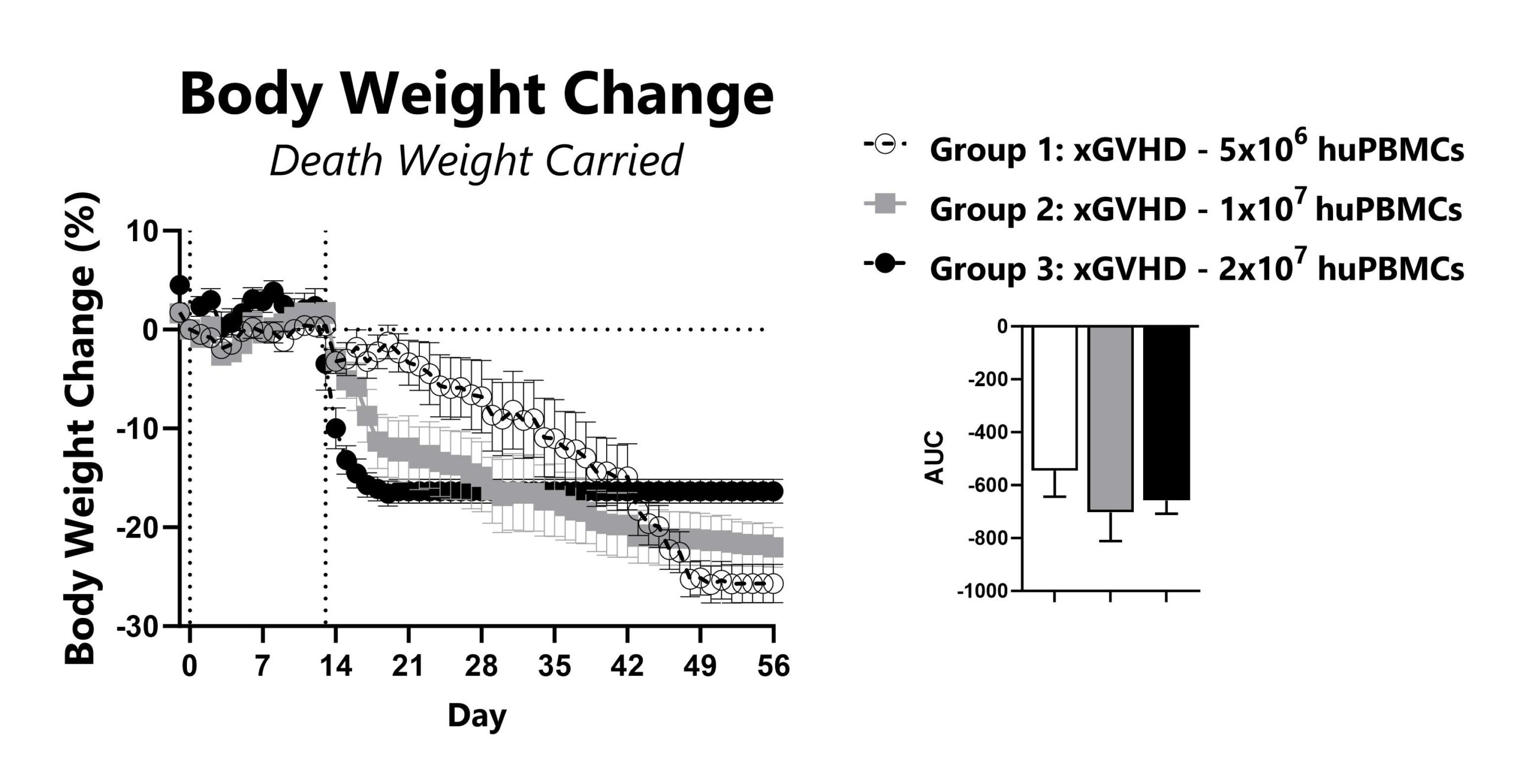
Recipient mice are pre-conditioned and transplanted with human (hu) PBMCs on Day 0. Animals are weighed daily, and body weight change as compared to Day 0 is calculated. To normalize for survivor bias, percent weight change with death weight carried forward is calculated. The AUC is calculated to compare treatment groups and is shown in the inset.
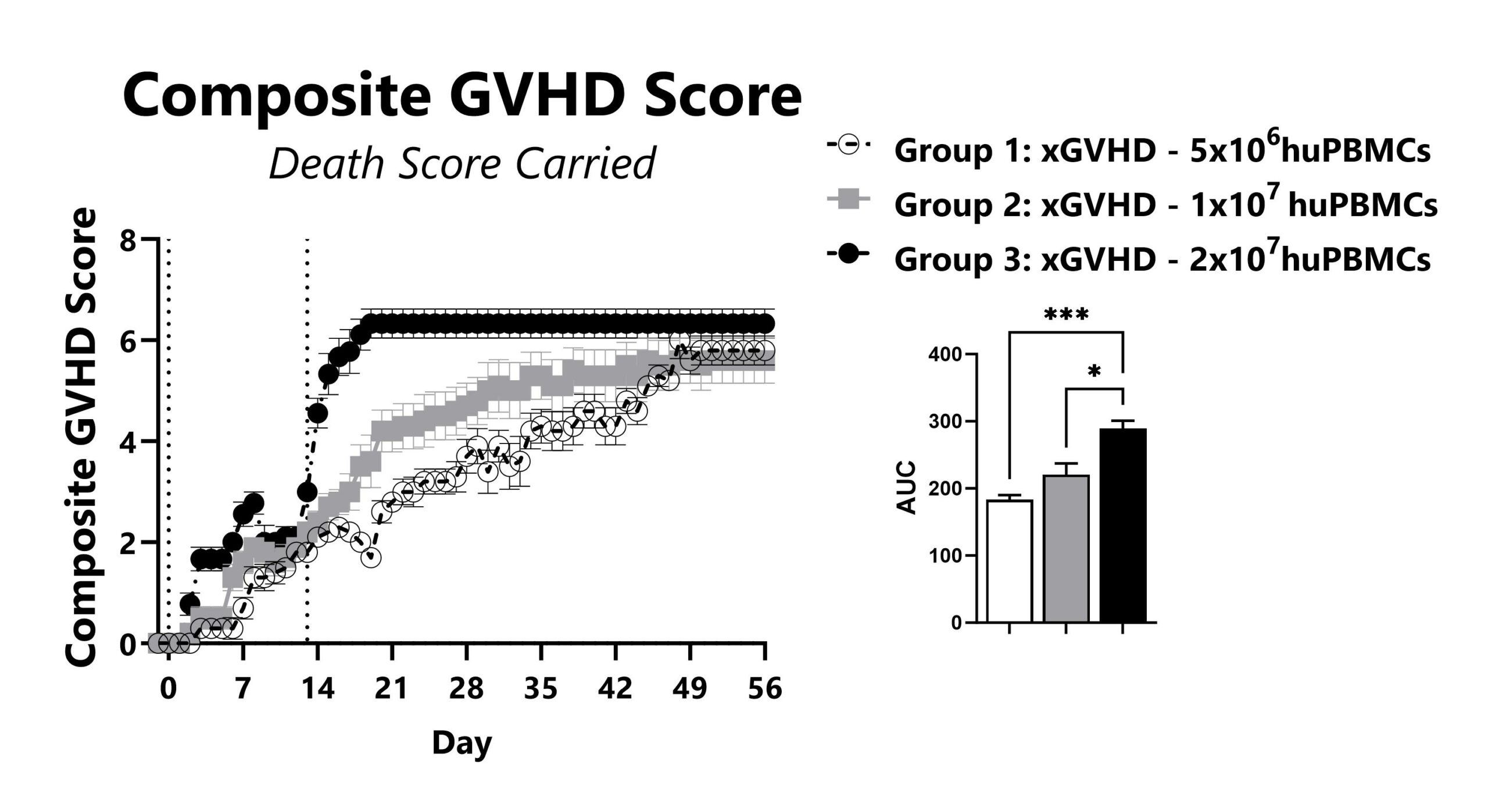
GVHD mice are assessed daily for weight loss, posture, activity, fur texture, and skin integrity phenotypes. Each phenotype is scored and used to calculate the composite GVHD score. To normalize for survivor bias, GVHD score with death score carried forward is calculated. The AUC is calculated to compare treatment groups and is shown in the inset. (*: p<0.05; ***: p<0.001)

Animals are assessed for survival and moribundity on a daily basis.
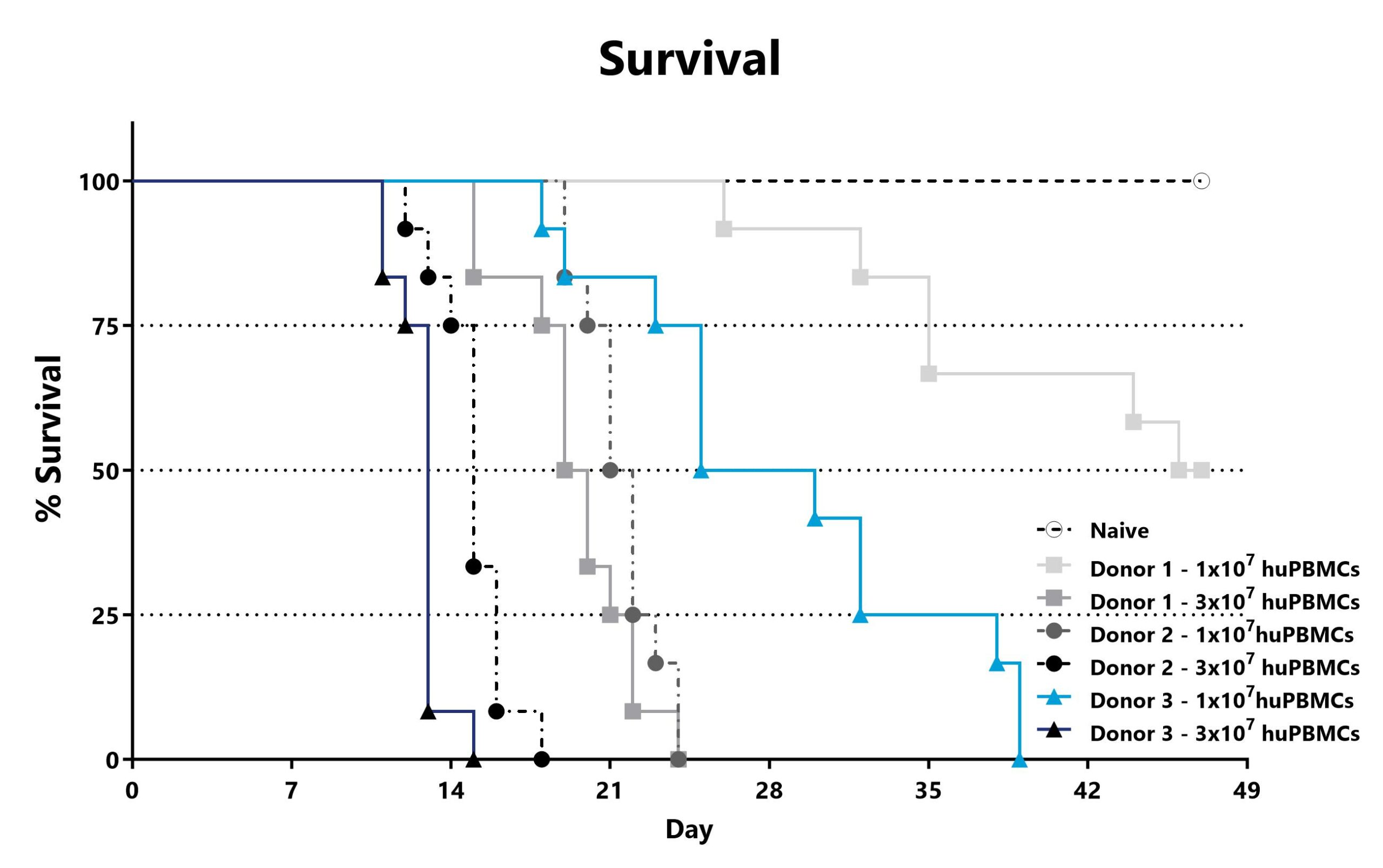
Recipient mice are pre-conditioned and transplanted with human (hu) PBMCs from different donors on Day 0. Animals are assessed for survival and moribundity on a daily basis.

Close

The H2b -> H2d major histocompatibility complex (MHC) mismatched model is also commonly used to investigate the GVL effect also referred to as graft vs tumor effect. The GVL effect is largely mediated by donor cytotoxic T-cell responses, and a goal of interventions has been to prevent GVHD while preserving GVL. Recipient mice (H2d) are pre-conditioned with a lethal dose of total body irradiation prior to the adoptive transfer of T-cell depleted bone marrow supplemented with splenocytes harvested from an MHC-mismatched donor mouse (H2b). Shortly after transplant, recipient mice are injected with bioluminescent tumor cells. When T-cells are present in the cell transplant, tumors fail to grow.
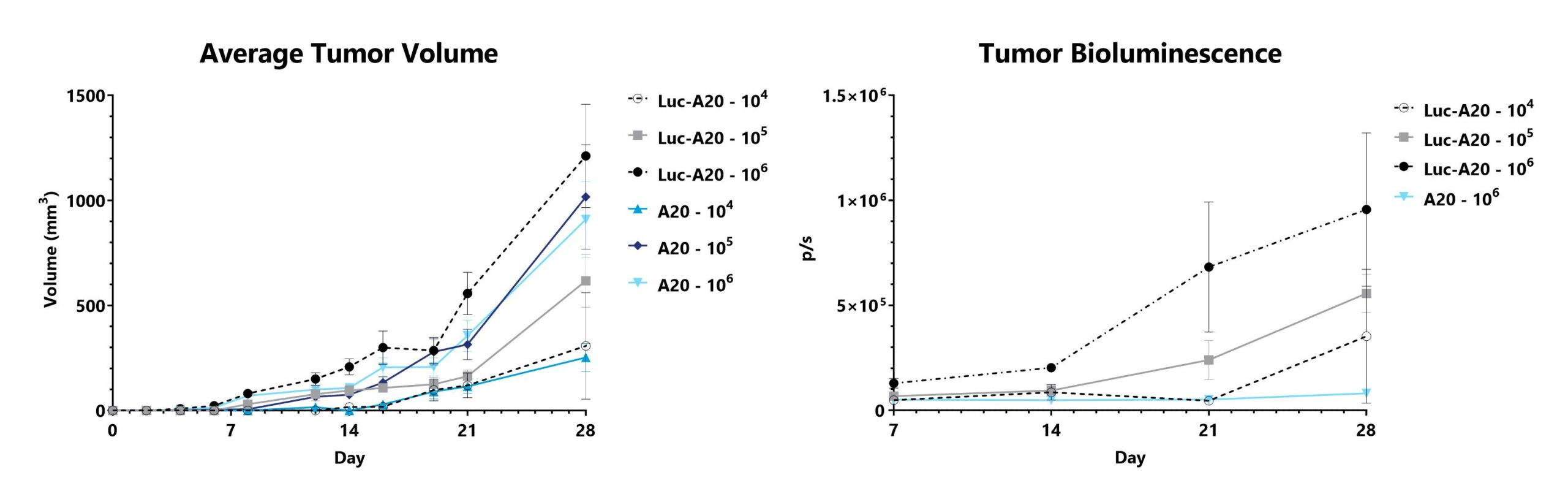
A20 cells or luciferase-expressing A20 cells (Luc-A20) are injected into recipient mice that receive a T-cell depleted bone marrow transplant. Tumor dimensions are measured and used to calculate tumor volume (left). Tumor bioluminescence is assessed using IVIS imaging (right).

Close
Study Models

The CIA model is one of the most widely studied and utilized models of arthritis as it shares immunological and pathological features with human rheumatoid arthritis (RA). Disease is induced in genetically susceptible rodents by immunization with chicken type II collagen emulsified in complete Freund’s adjuvant (CFA), followed by an additional immunization with chicken type II collagen emulsified in incomplete Freund’s adjuvant (IFA). After the second injection, LPS is given to synchronize the onset of arthritis. This paradigm results in inflammation of the paws which is evaluated over the course of the study using an established scoring scale, in addition to monitoring paw edema through caliper measurement. The immunopathogenesis of CIA involves both T and B cell-specific responses to type II collagen. Corticosteroids and biologics are effective in treating disease and can be used as positive comparator arms.
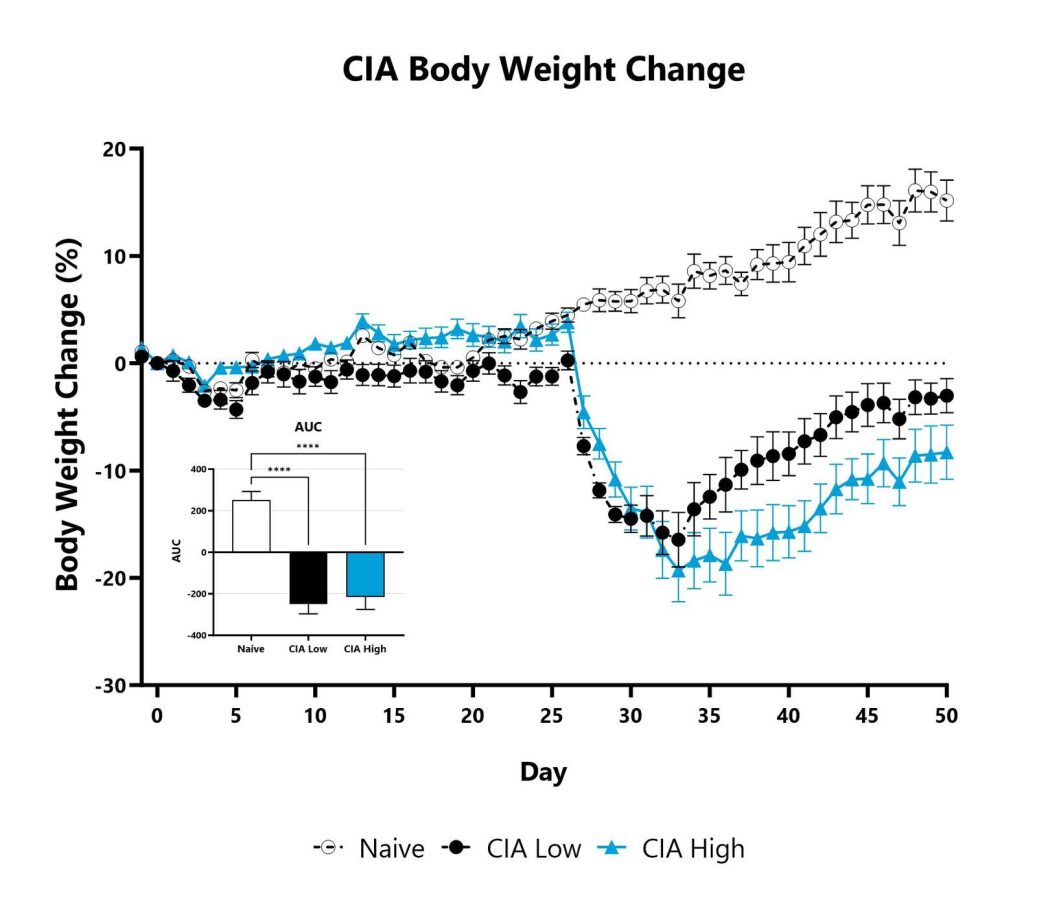
Animals are weighed daily, and body weight changes as compared to Day 0 are calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (****p<0.001)
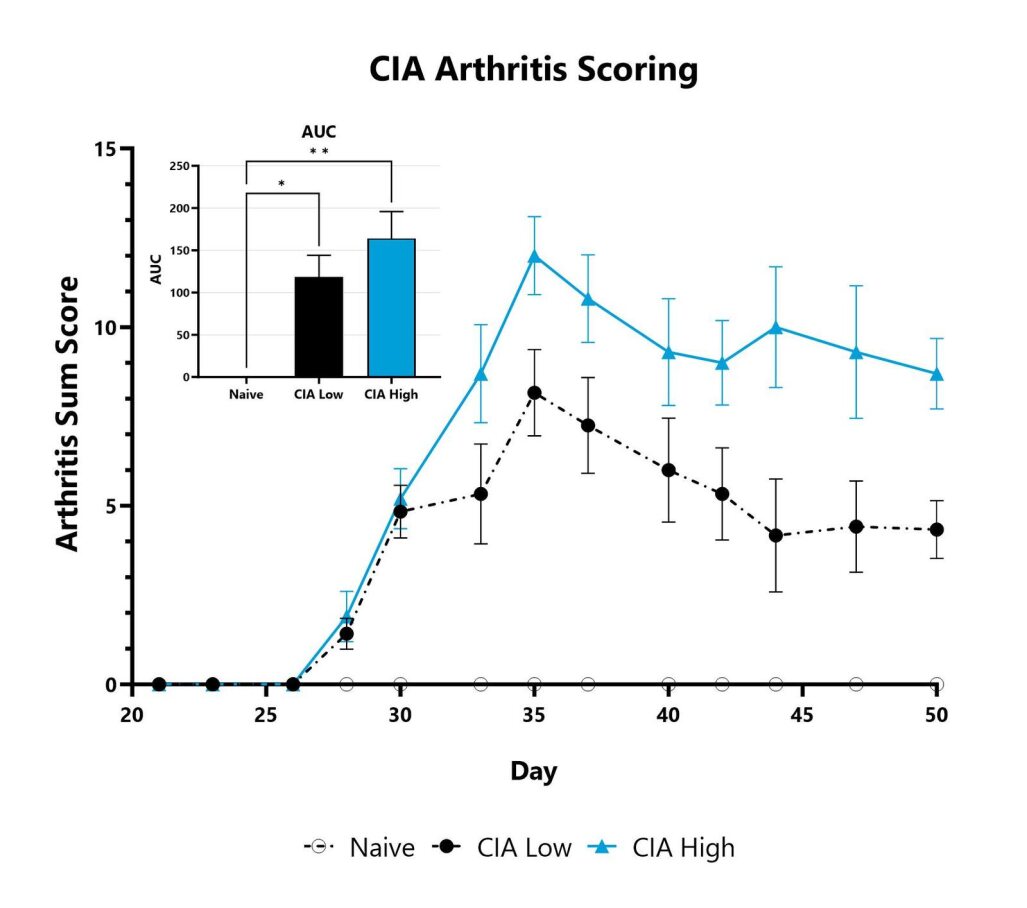
Mice with CIA are assessed three times per week for clinical arthritis development. Each paw is scored on a scale of 0-4, and the sum score is shown. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05; **p<0.01)
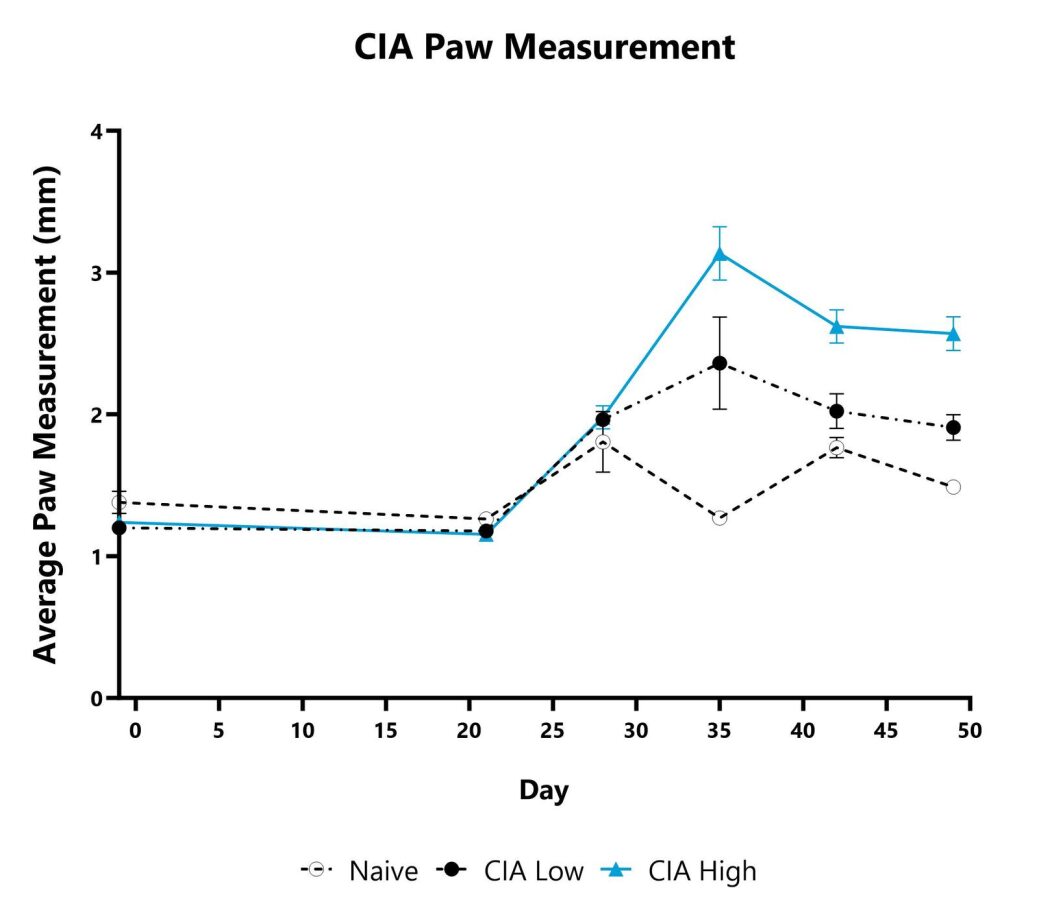
Paw swelling/edema is assessed weekly in the mouse CIA model by measurement with a digital caliper. Each paw is measured, and the average is presented for each animal.

Close

The CAIA model is a widely used pre-clinical rodent model mimicking many of the pathophysiological features of CIA and RA. This model offers some key advantages over the traditional CIA model including a short disease induction time, induction of disease in various mouse strains (not only susceptible strains), and a shorter study duration in which to screen potential therapeutics. Arthritis is induced through injection of monoclonal antibodies that target type II collagen, followed by administration of LPS to synchronize the onset of disease. Potential therapeutics that target the downstream inflammatory processes involved in arthritis (not requiring the presence of antigen-specific T and B cells) are applicable in this model. Corticosteroids and biologics are effective in treating disease and can be used as positive comparator arms.
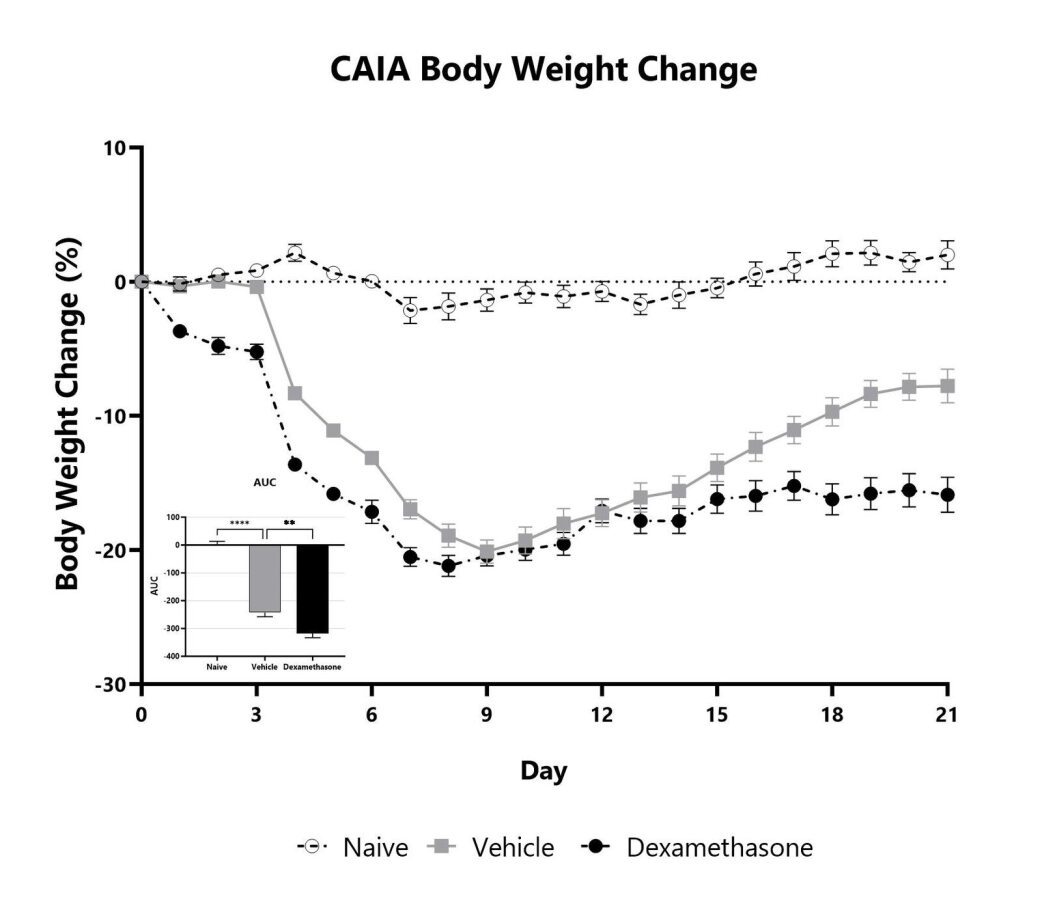
Animals are weighed daily, and body weight changes as compared to Day 0 are calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (**p<0.01; ****p<0.001 compared to vehicle control)
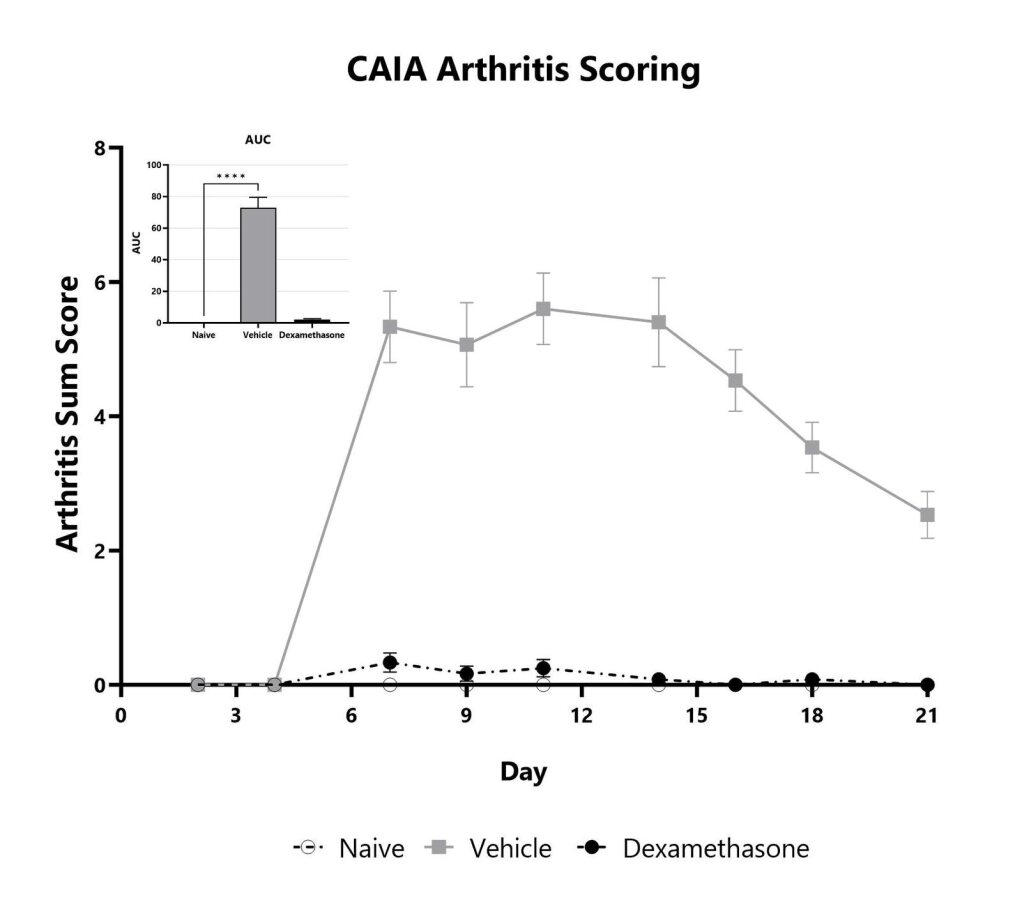
Mice with CAIA are assessed for clinical arthritis development over the course of the study. Each paw is scored on a scale of 0-4, and the sum score is shown. The AUC is calculated to compare treatment arms and is shown in the inset. (****p<0.001)
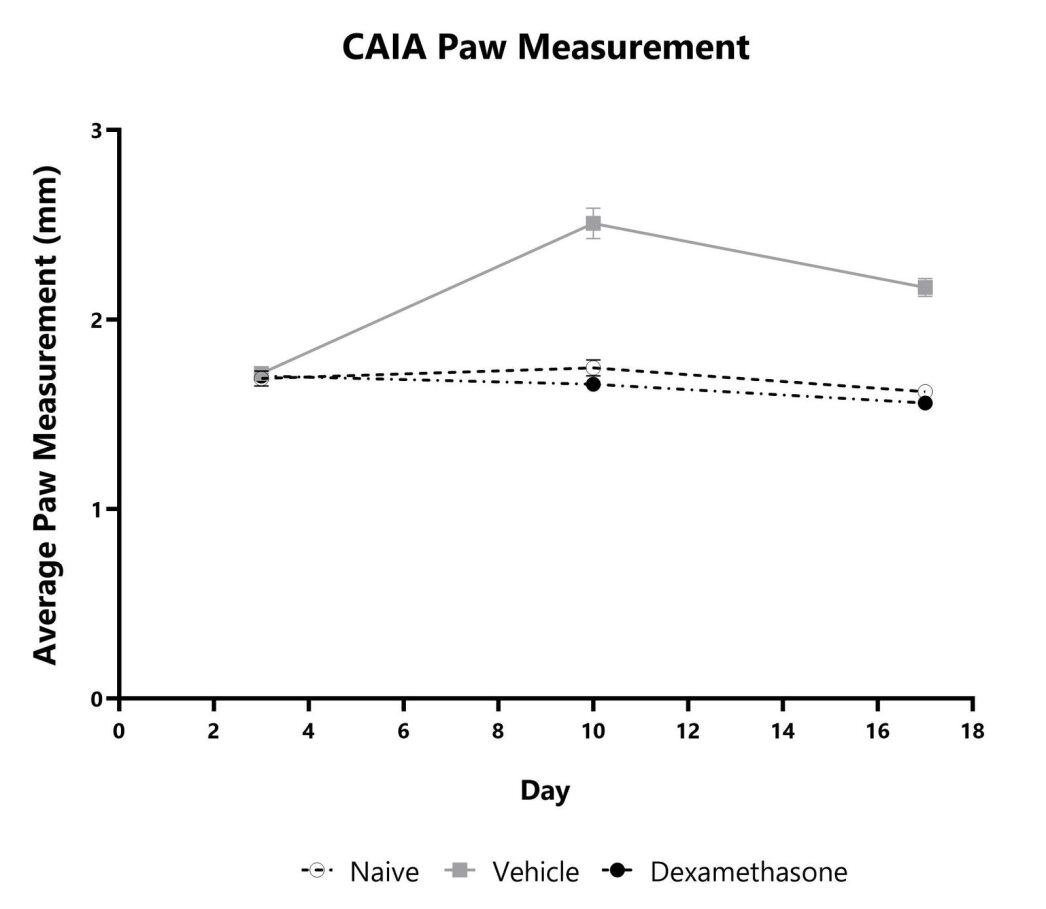
Paw swelling/edema is assessed weekly in the mouse CAIA model by measurement with a digital caliper. Each paw is measured, and the average is presented for each animal.

Close

BioModels offers a model of surgically induced OA in rats that is commonly used to test potential therapies to improve joint pain and prevent subsequent deterioration of the joint. Disease is induced by surgically transecting the anterior cruciate ligament (ACL) without damaging the meniscus. The animals are allowed to move freely, and inflammation causes damage to the joint over time. OA is typically apparent beginning 4-5 weeks after induction. The rat OA model (unilateral ACL transection) relates to sports injuries in humans that can often occur where the ACT is damaged or severed, but the underlying disease processes are comment to all joints affected by OA. Treatments are often injected directly into the affected joint.
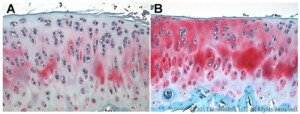
Representative Safranin O stained rat articular cartilage 36 days following ACL transection. (A) PBS-treated, (B) Treated with intra-articular Enbrel injections that began 7 days after surgery and occurred twice weekly.
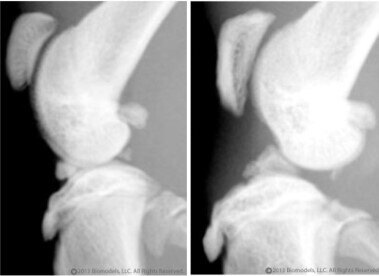
Lateral radiograph on left is from a normal rat, lateral radiograph on right is from a rat 32 days after of ACL transection.

Close
Study Models

The MOG35-55 model of EAE is considered the gold standard model in the field and is routinely used in the development of therapeutics for MS. Phenotypes are mediated by self-reactive encephalitogenic T cells, as well as macrophages, resident microglia, and astrocytes. Disease is induced by immunization with a myelin oligodendrocyte glycoprotein (MOG) emulsified in Complete Freund Adjuvant (CFA), followed by Pertussis toxin administration. An ascending flaccid paralysis that begins with a limp tail and progresses to hind and forelimb paralysis is observed. Test article responses can be compared to the effects of Prednisolone or Fingolimod.
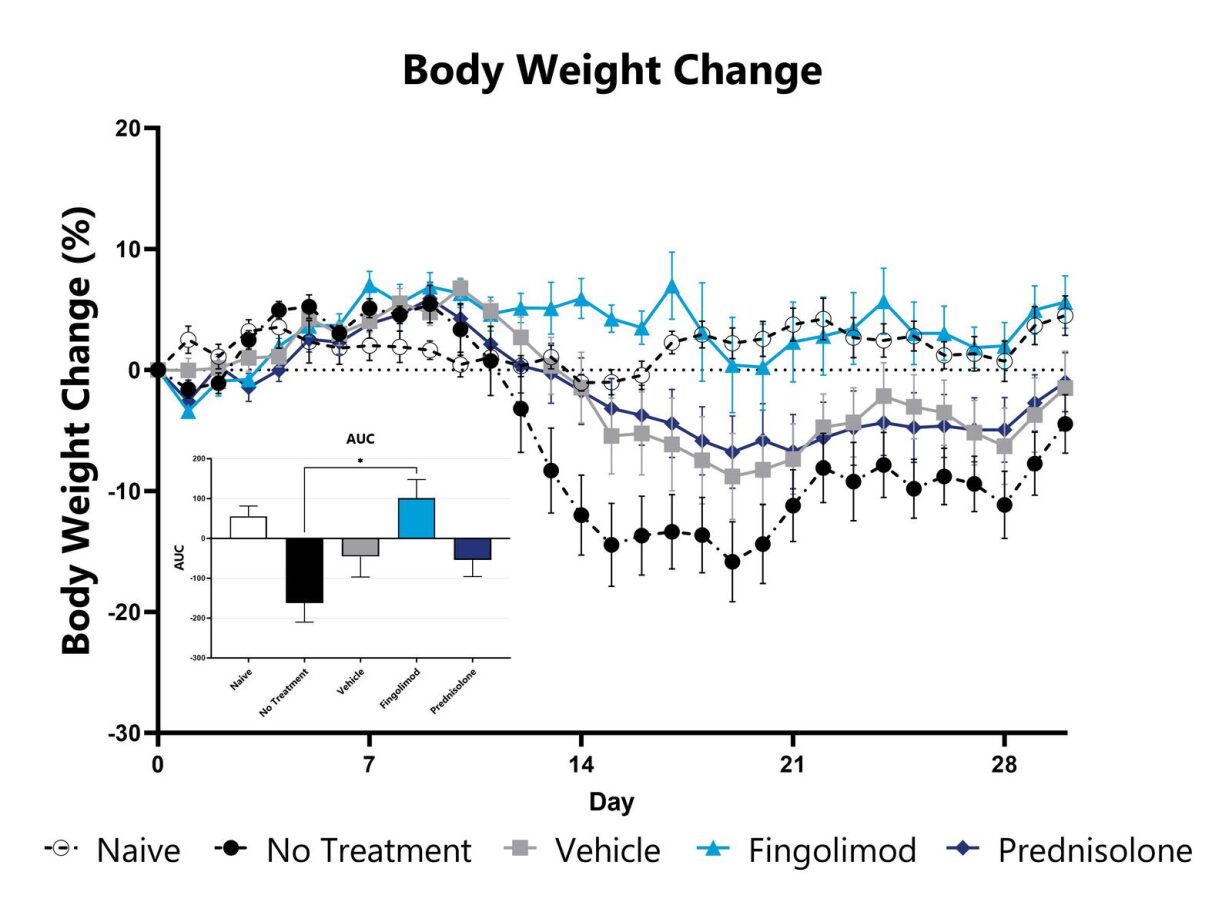
MOG-induced EAE animals are weighed daily, and body weight compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05 compared to the vehicle-control)
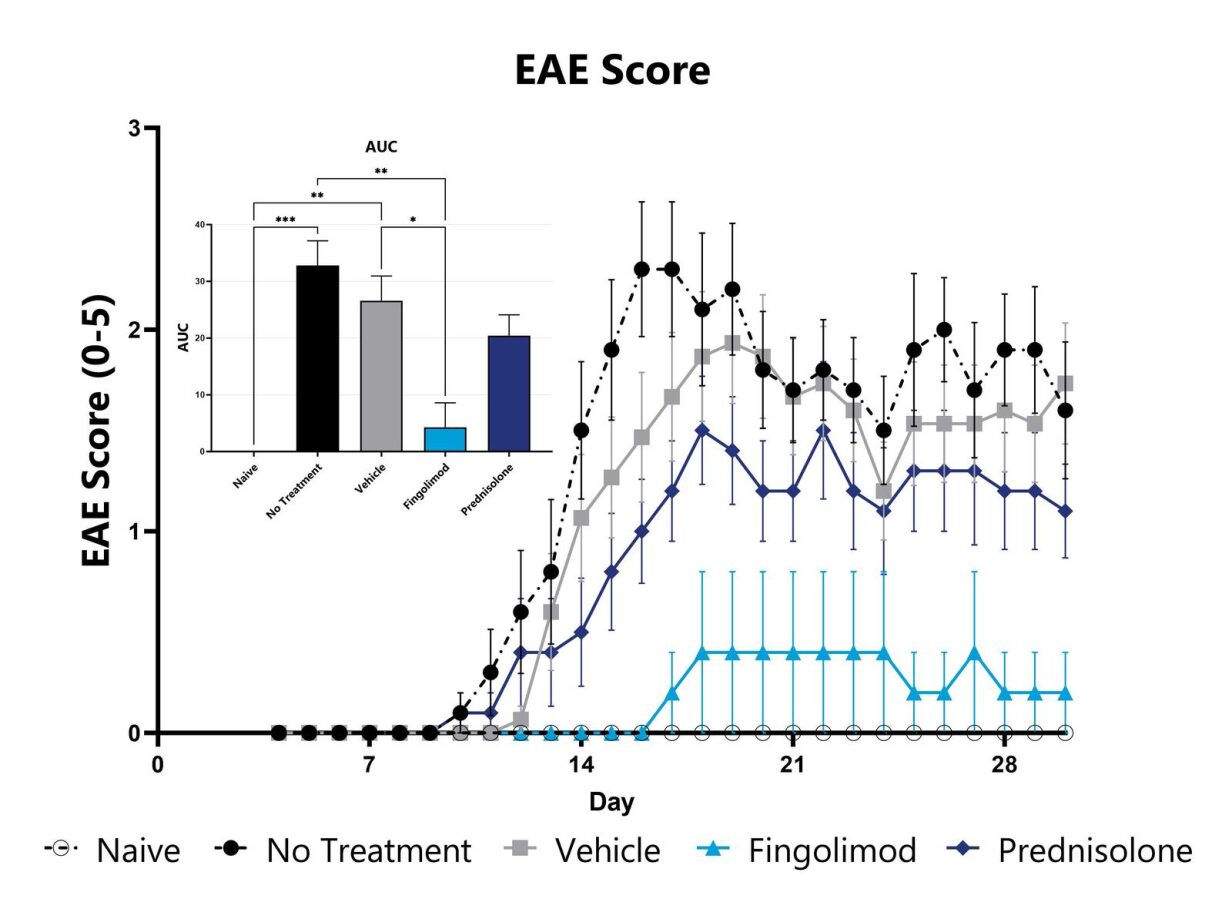
Animals are evaluated for clinical signs of EAE on a daily basis and are assigned an EAE score. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05; **p<0.01; ***p<0.005 compared to the vehicle-control)
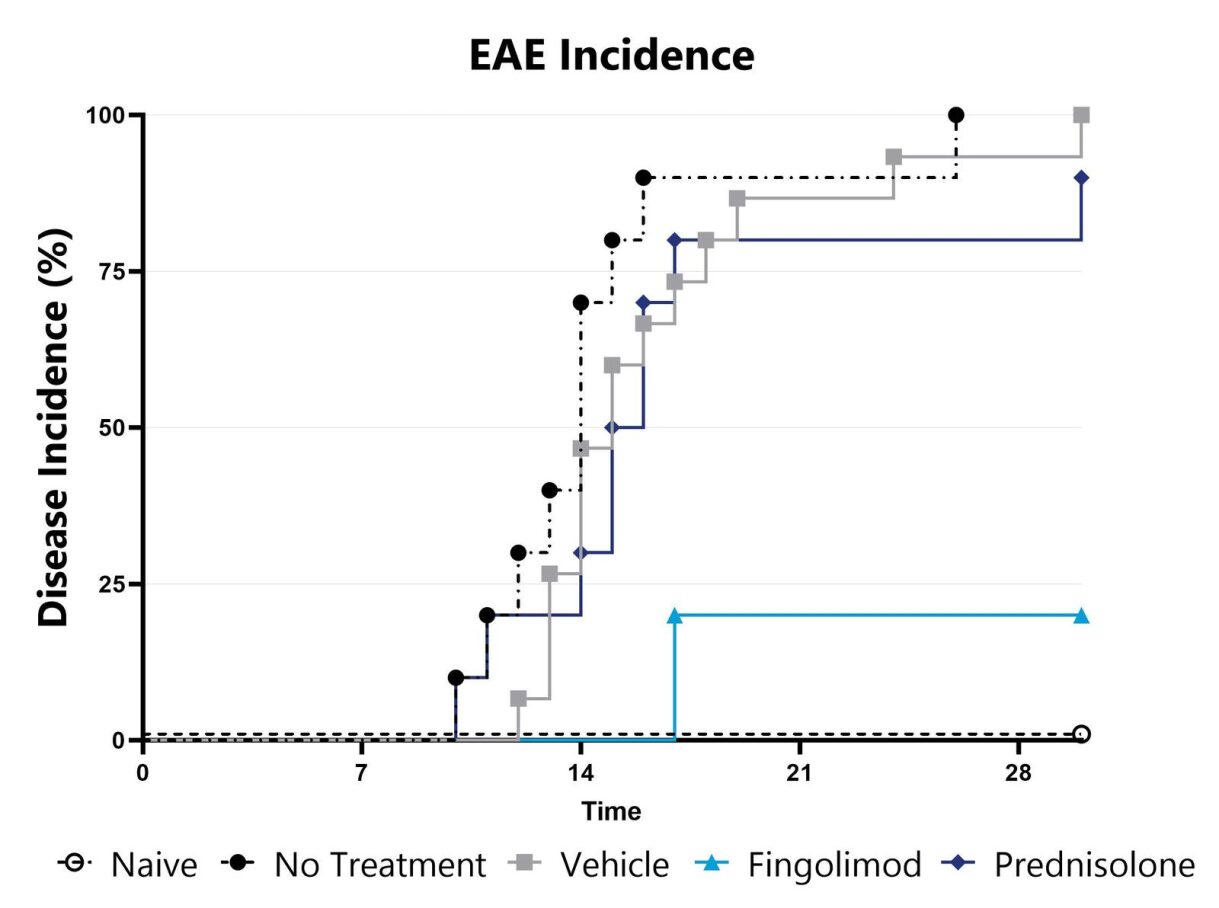
Animals are evaluated for clinical signs of EAE on a daily basis and the overall disease incidence is calculated.

Close


Close
Study Models

The lipopolysaccharide (LPS) acute sepsis model is routinely utilized to evaluate the sepsis phenotype of systemic inflammatory response syndrome. A hallmark of sepsis is the widespread elevation of cytokines. In rodents, LPS elicits a strong immune response within hours following administration. In the acute model, this response is measured by examining IL-6, TNF-α, or other cytokine concentrations in blood plasma/serum. Compounds which prevent the elevation of cytokines are predicted to be efficacious for sepsis.
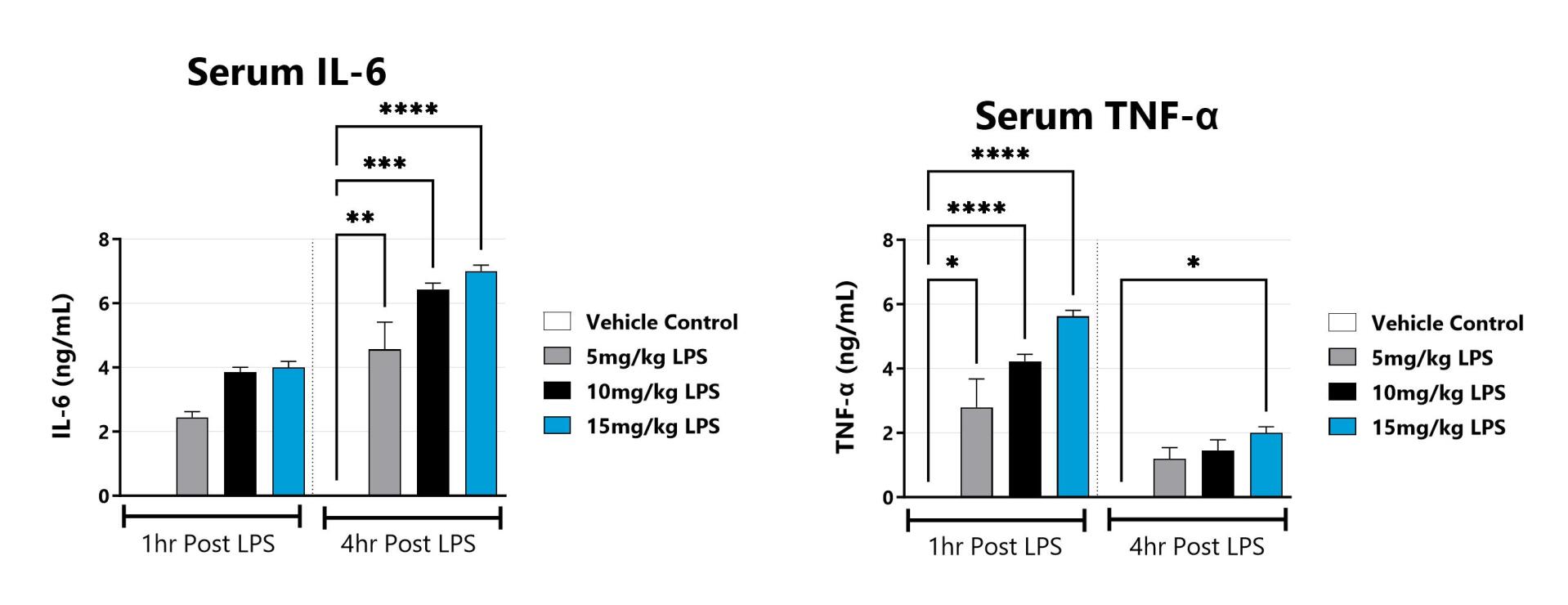
Blood is collected at 1hr and 4hrs post LPS administration and evaluated for a panel of cytokines including IL-6, and TNFα. (*p<0.05; **p<0.01, ***p<0.001, ****p<0.0001 as compared to vehicle control)

Close

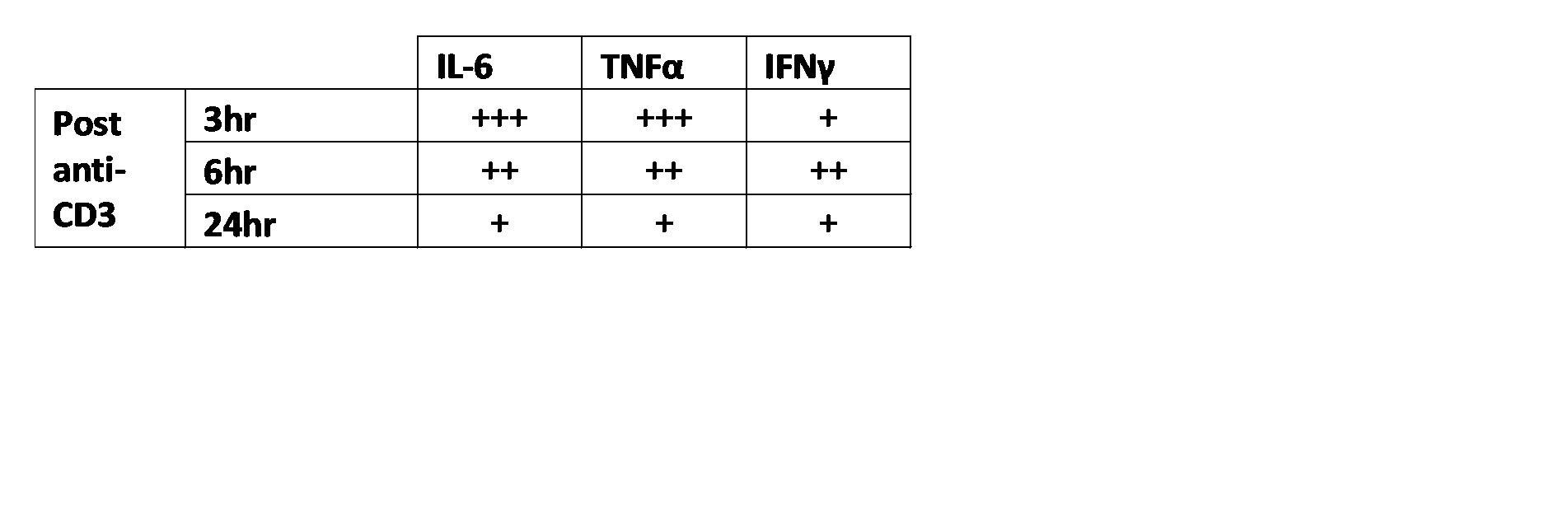
The anti-CD3 induced cytokine release syndrome (CRS) model is commonly used to evaluate T cell mediated cytokine storm. Similar to sepsis, disease is characterized by upregulation of pro-inflammatory cytokines. In rodents, anti-CD3 elicits a strong immune response within hours following administration. This response is measured by examining IFN-γ, IL-6, TNF-α, or other pro-inflammatory cytokine concentrations in blood plasma/serum. Compounds which prevent the elevation of cytokines are predicted to offer protection against CRS.
Animals with OXZ-induced colitis are weighed daily, and body weight change as compared to baseline is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (**p<0.01 compared to the Naive).

Close

The NOD2 agonist-induced peritonitis model is commonly used to evaluate MAPK and NF-κB mediated cytokine release. Similar to sepsis, disease is characterized by upregulation of pro-inflammatory cytokines. In rodents, NOD2 agonist (L18-MDP) elicits a strong immune response within hours following administration. This response is measured by examining IL-6, KC, TNF-α, or other pro-inflammatory cytokine concentrations in blood plasma/serum.
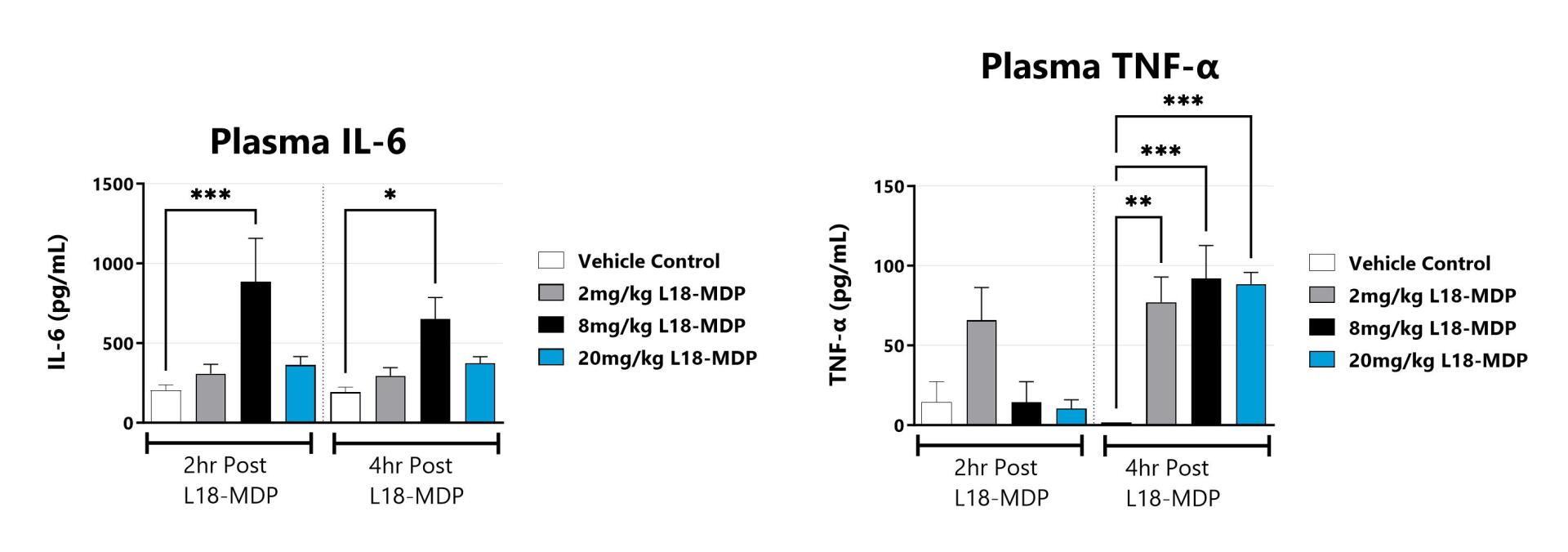
Blood is collected at 2hrs and 4hrs post L18-MDP administration and analyzed for a panel of cytokines including IL-6, and TNFα. (*p<0.05; **p<0.01, ***p<0.001 as compared to vehicle control)

Close

The rIL-2 induced vascular leak syndrome (VLS) model is often used to recapitulate IL-2 toxicity associated with immunotherapy. Manifestations of disease include transendothelial migration of inflammatory cells into the lung and liver which leads to organ injury. In rodents, rIL2 elicits a strong immune response within hours following administration. This response is measured by examining lung and liver vascular permeability by Evan’s blue assays, and complete blood cell (CBC) counts in blood.
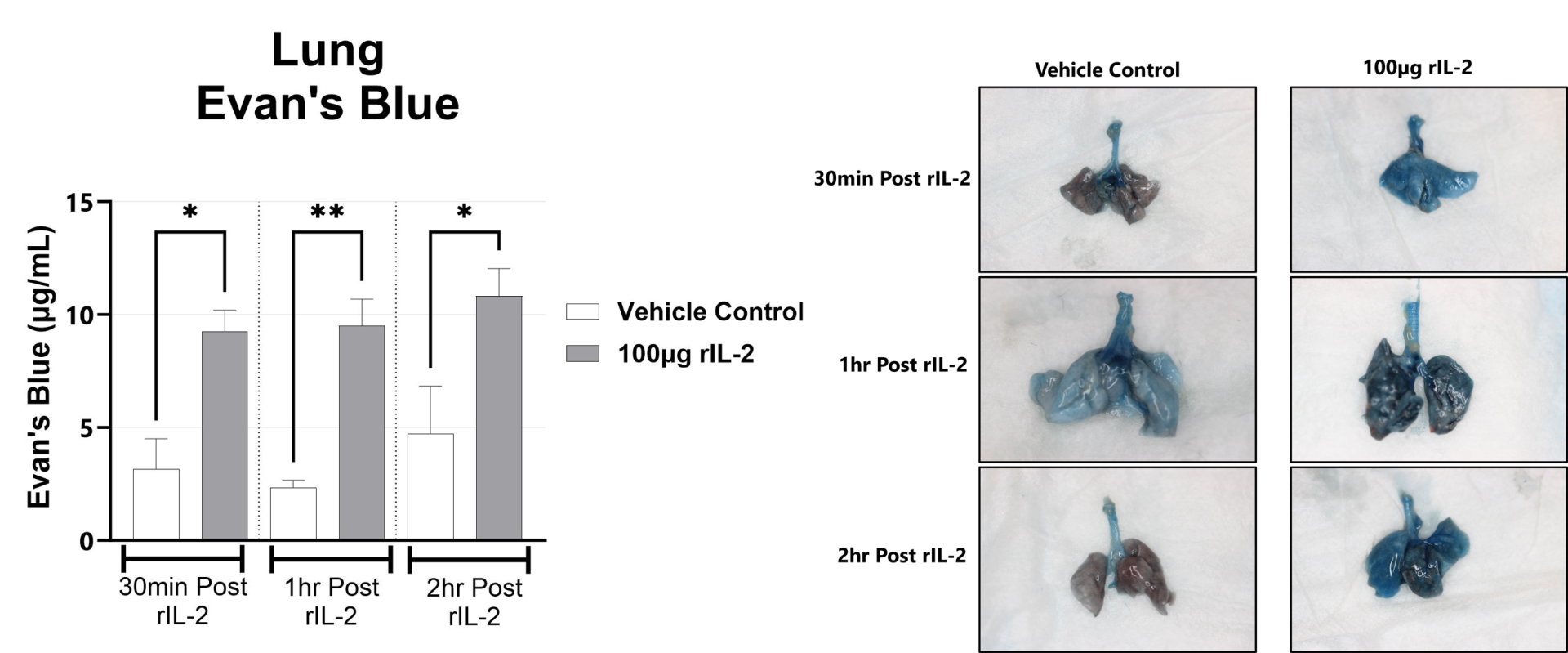
Following rIL-2 administration, Evans Blue dye localization in the lungs is assessed by measuring the absorbance at 650nm in the tissue. (*p<0.05; **p<0.01 as compared to vehicle control). Representative images of the lungs from each group are shown.
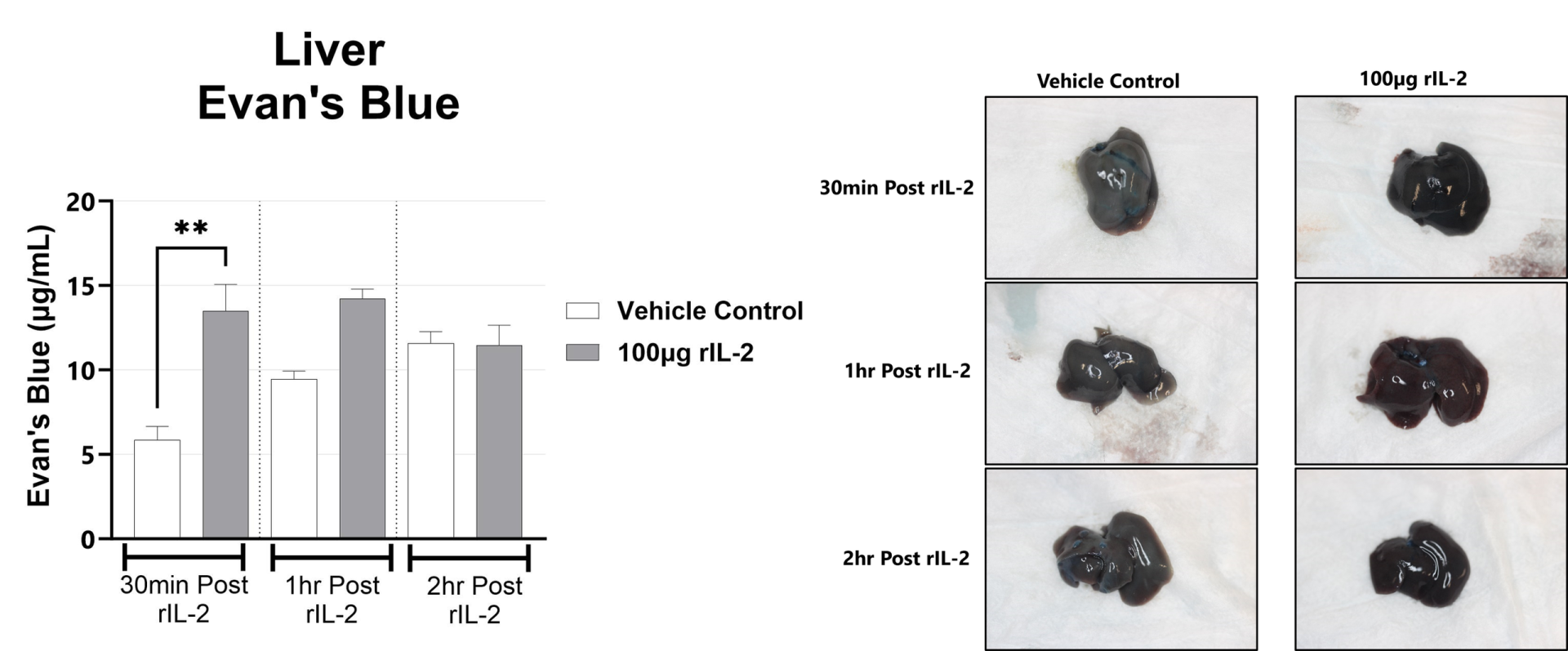
Following rIL-2 administration, Evans Blue dye localization is in the liver is assessed by measuring the absorbance at 650nm in the tissue. (**p<0.01 as compared to vehicle control). Representative images of the liver from each group are shown.
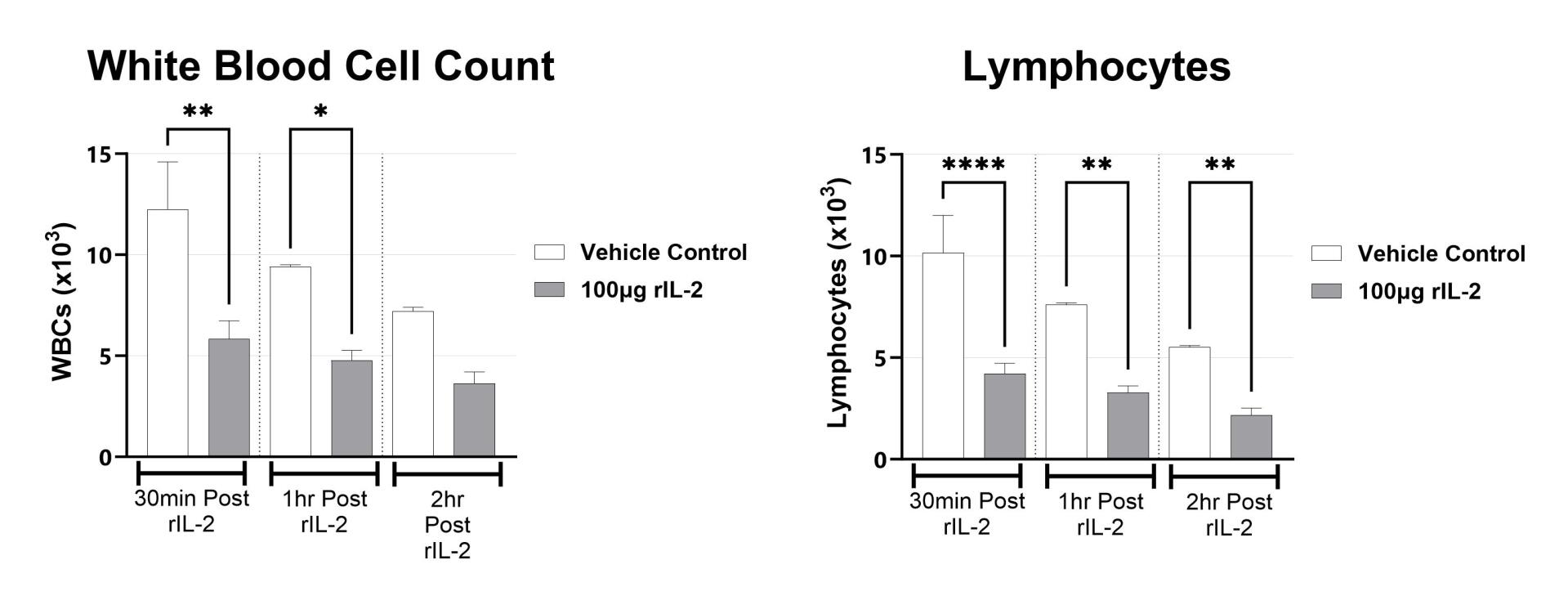
Following rIL-2 administration, blood is collected upon euthanasia and Complete Blood Cell Count is performed. Mean group values for WBC and Lymphocyte counts are shown. (*p<0.05; **p<0.01, ****p<0.0001 as compared to vehicle control)

Close
Study Models

BioModels offers a mouse model of ovalbumin (OVA) induced atopic dermatitis. Atopic dermatitis is a condition that causes dry, itchy, and inflamed skin and affected patients are at increased risk of developing other conditions such as food allergies, hay fever, and asthma. Dermatitis is induced via epicutaneous OVA sensitization. Elevated OVA-specific IgE, IgG1 and IgG2a are observed. Test article responses can be compared to the effects of Dexamethasone.
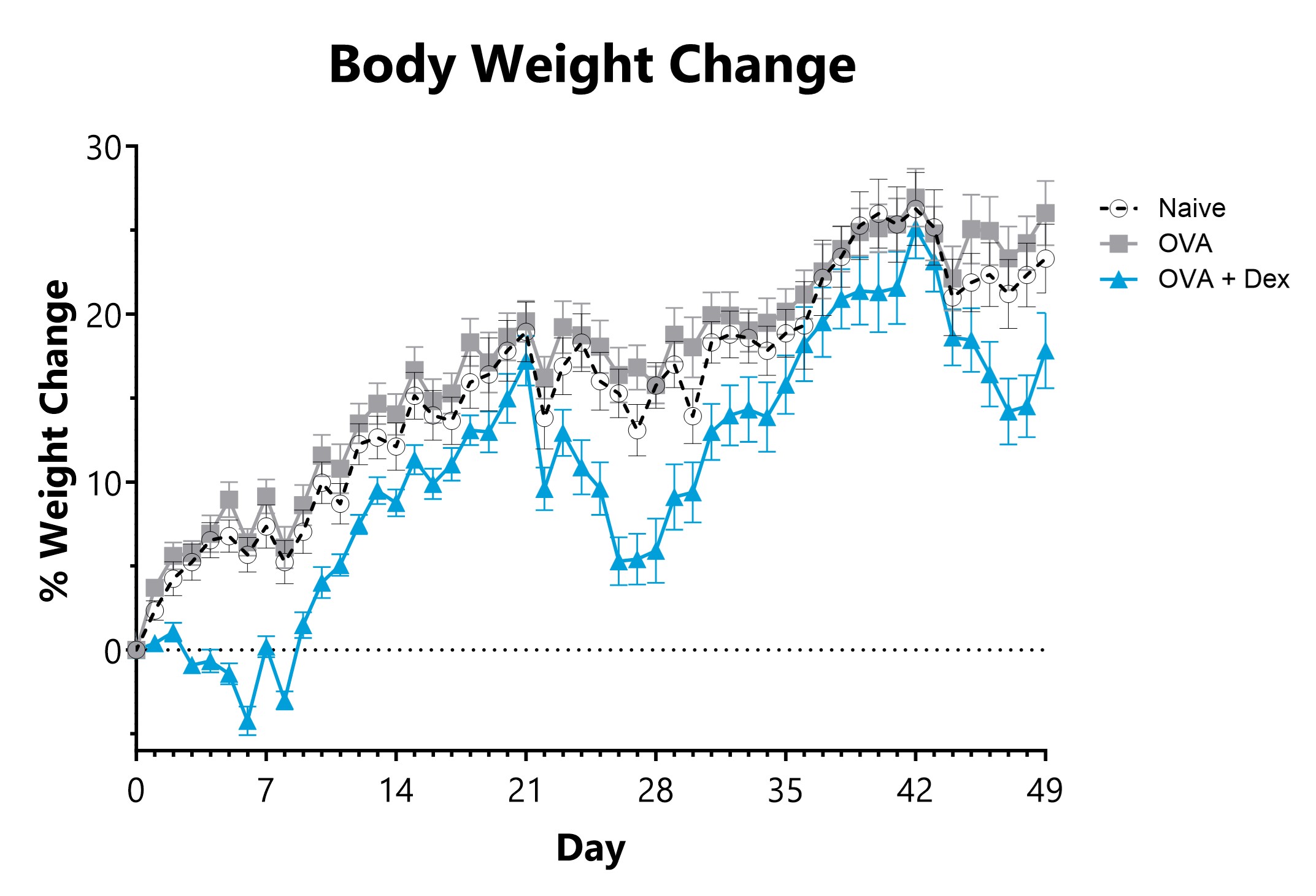
Epicutaneous OVA-sensitized animals are weighed daily, and body weight change relative to Day 0 is calculated.
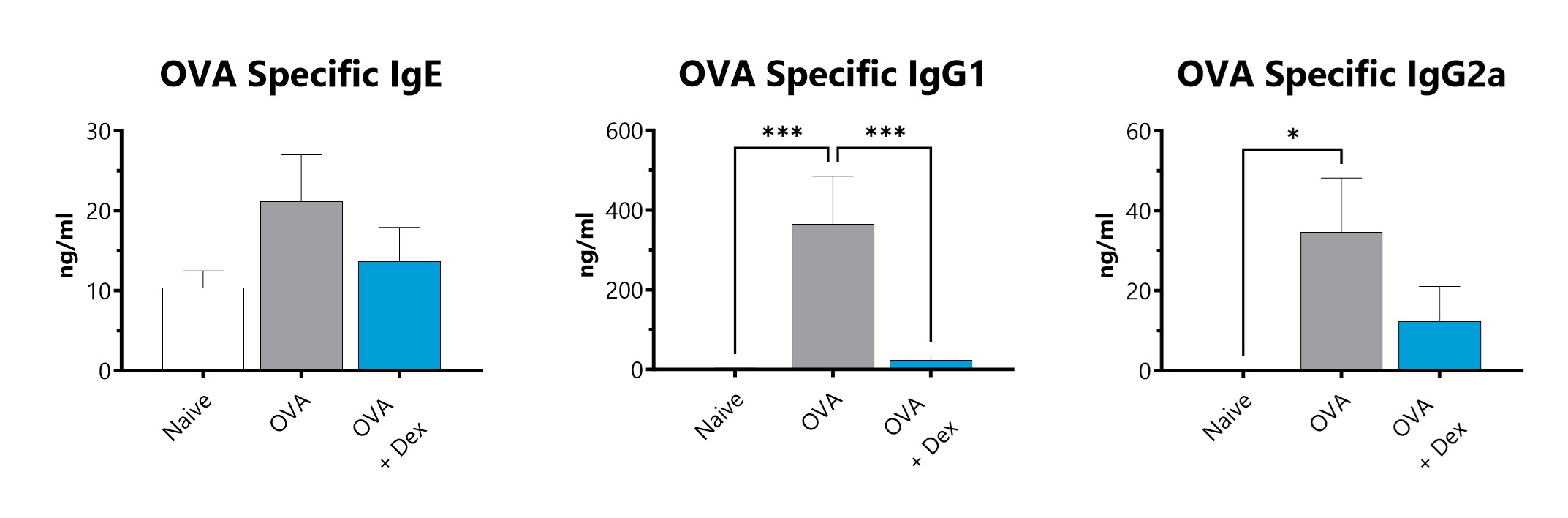
Serum is collected and assessed for OVA-specific IgE, IgG1, and IgG2 by ELISA. (*p<0.05; ***p<0.001 compared to the naïve-control).

Close

At BioModels, you can assess your novel therapeutics for efficacy and mechanism of action in our established radiation-induced dermatitis model to address unmet clinical needs for oncology patients. Dermatitis is an inflammatory condition of the skin that occurs as a result of exposure to radiation during treatment for a variety of malignancies. Ionizing radiation induces DNA damage that leads to cell death in addition to the generation of reactive oxygen species (ROS). At BioModels, radiation dermatitis is induced in mice with either a single bolus of acute radiation, or with 6 fractionated, smaller doses, targeted to the skin. Animals are monitored daily and evaluated for overall health and survival in addition to dermatitis severity over the course of the study. Peak disease differs based on the method of induction, with dermatitis persisting through the final evaluation which can then include histopathology.
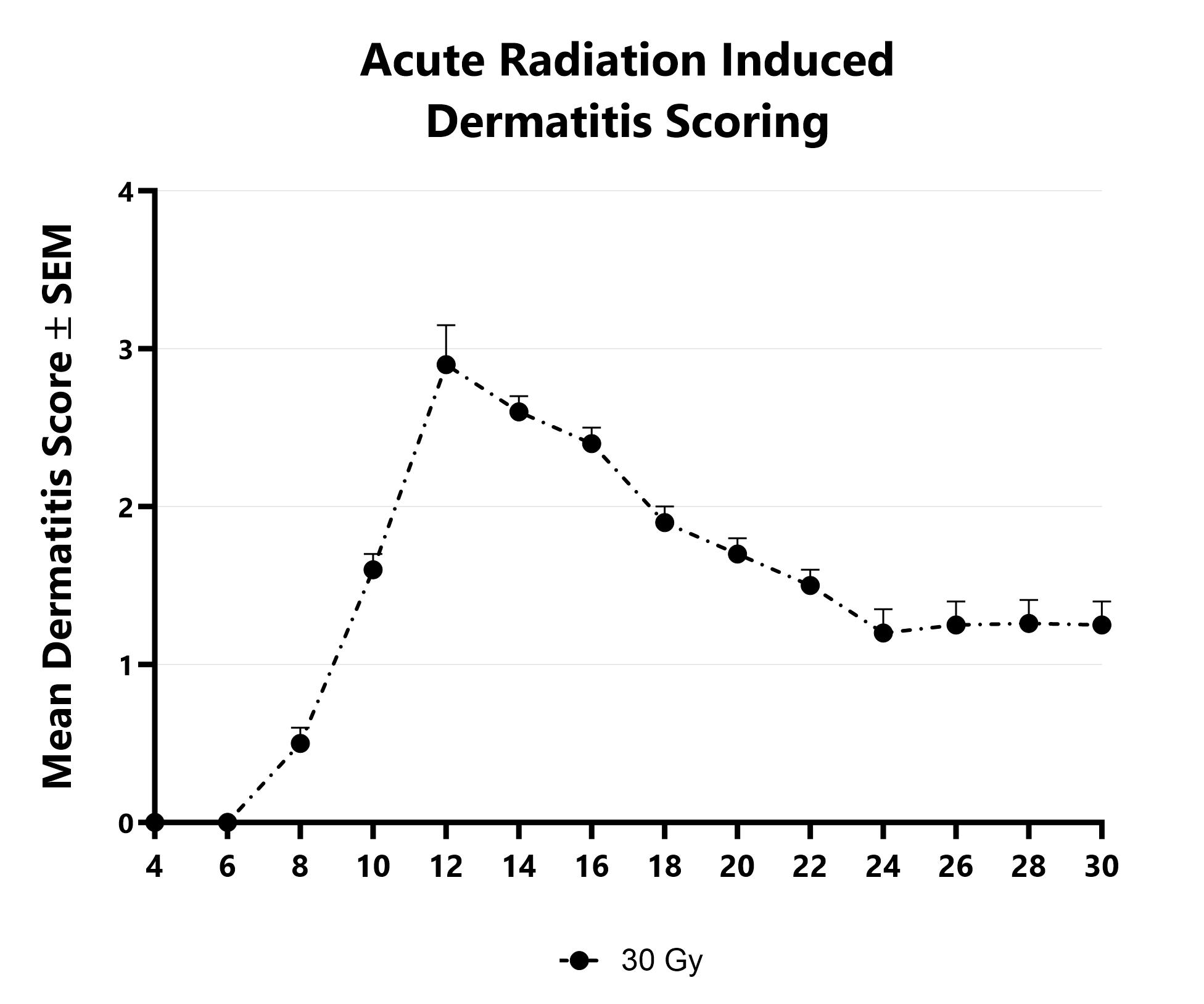
Dermatitis severity is assessed longitudinally using an established scoring scale at multiple timepoints during an acute radiation-induced dermatitis study.
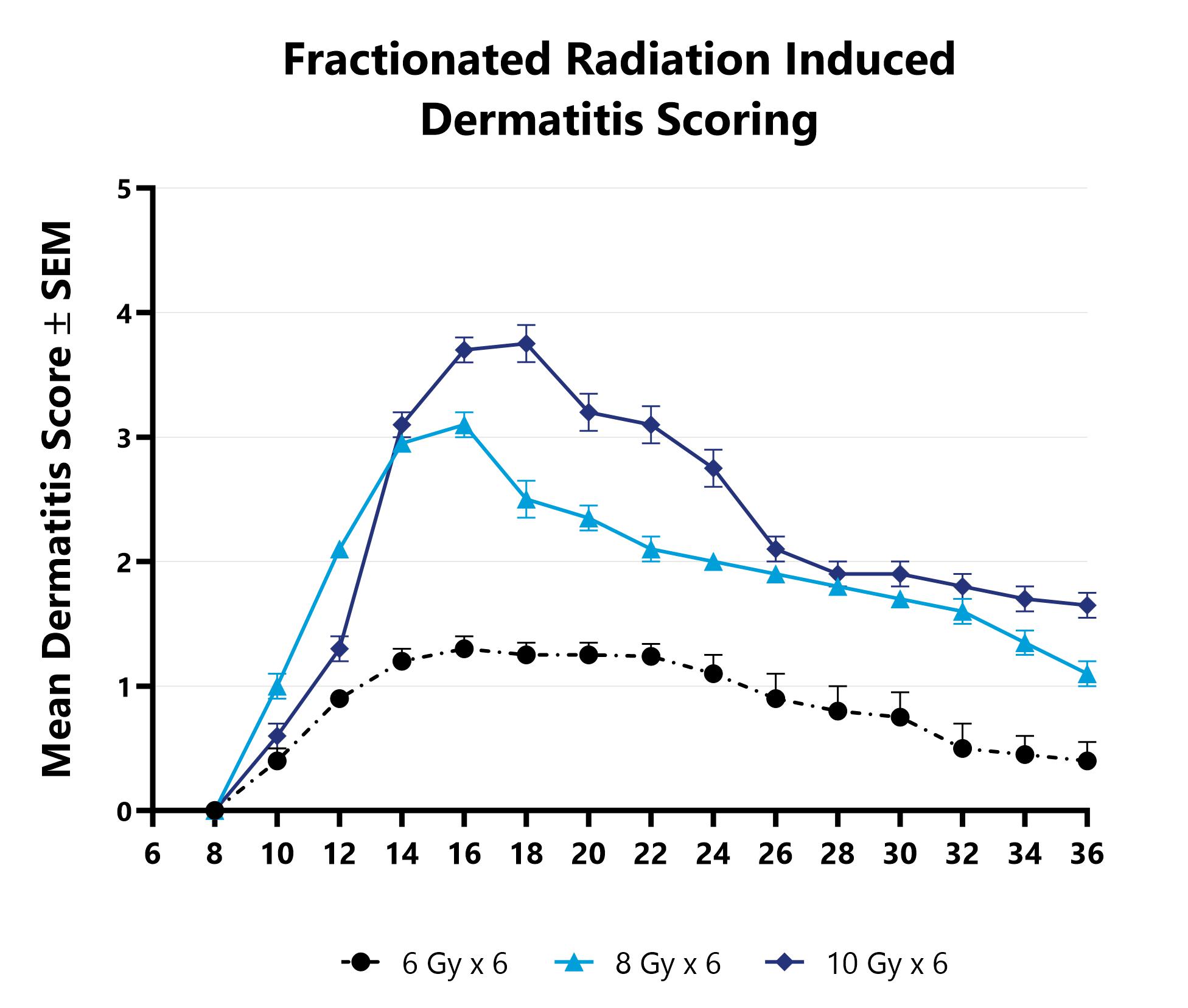
Dermatitis severity is assessed longitudinally using an established scoring scale at multiple timepoints during an acute radiation-induced dermatitis study.

Close

At BioModels, you can assess your novel therapeutics for efficacy and/or mechanism of action in our established UVB radiation-induced dermatitis model. Ultraviolet radiation from the sun or other sources is a primary cause of skin damage, leading to sunburn, erythema, blistering, and melanoma in cases of chronic exposure. UVB rays can penetrate the epidermal layer of the skin causing cellular and DNA damage. BioModels’ UVB skin damage model uses precise dosing of UV radiation with a well-established scoring system and selected histological markers to quantify damage levels to specific skin cells/layers. Dermatitis is induced in mice using a single or multiple doses of UVB radiation directed to the dorsal skin. Animals are monitored daily and evaluated for overall health and survival in addition to skin damage severity over the course of the study. The main endpoints include clinical skin damage assessment in addition to histopathological evaluation of the affected skin.
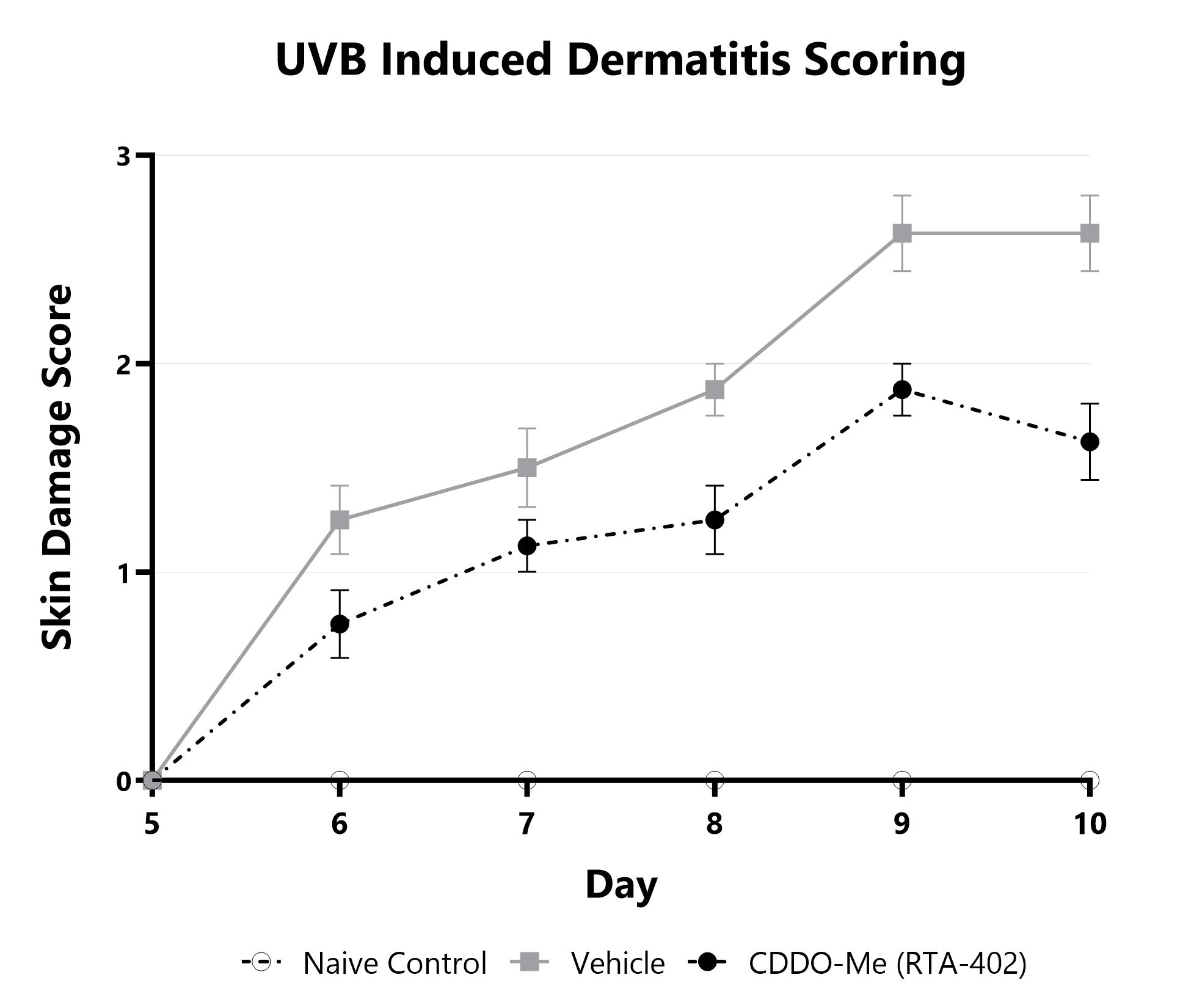
Skin damage severity is assessed longitudinally using a pre-established scoring scale at multiple timepoints during the UVB induced dermatitis model. Naïve, vehicle-treated, and CDDO-Me (RTA-402)-treated groups are shown.

Close
Study Models

BioModels offers a mouse model of ovalbumin (OVA) induced allergic asthma. In the acute model, mice are sensitized with an intraperitoneal (IP) injection of OVA and adjuvant and then challenged with intranasal delivery of OVA followed by collections on day 16. Chronic versions of this model run from 4-12 weeks. The OVA model recapitulates many of the hallmarks of allergic asthma in humans, including elevated IgE and Th2 related cytokines, mucus hypersecretion, airway inflammation, goblet cell hyperplasia, epithelial hypertrophy, and airway hyperreactivity. Endpoints in this model include total and differential cell counts and inflammatory mediator content in the broncho-alveolar lavage fluid, airway hyperreactivity and detailed lung mechanics, as well as histopathology and immunohistochemistry.
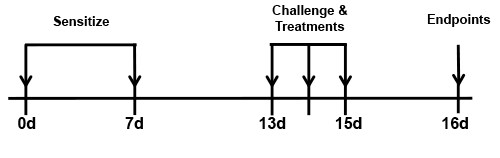
BALB/c mice are sensitized with an IP injection of OVA with adjuvant on days 0 and 7. Mice are then challenged with OVA intranasally on days 13, 14, and 15 and endpoints are assessed on day 16. Reference treatment animals receive 3 mg/kg dexamethasone 1 hour prior to the challenges.
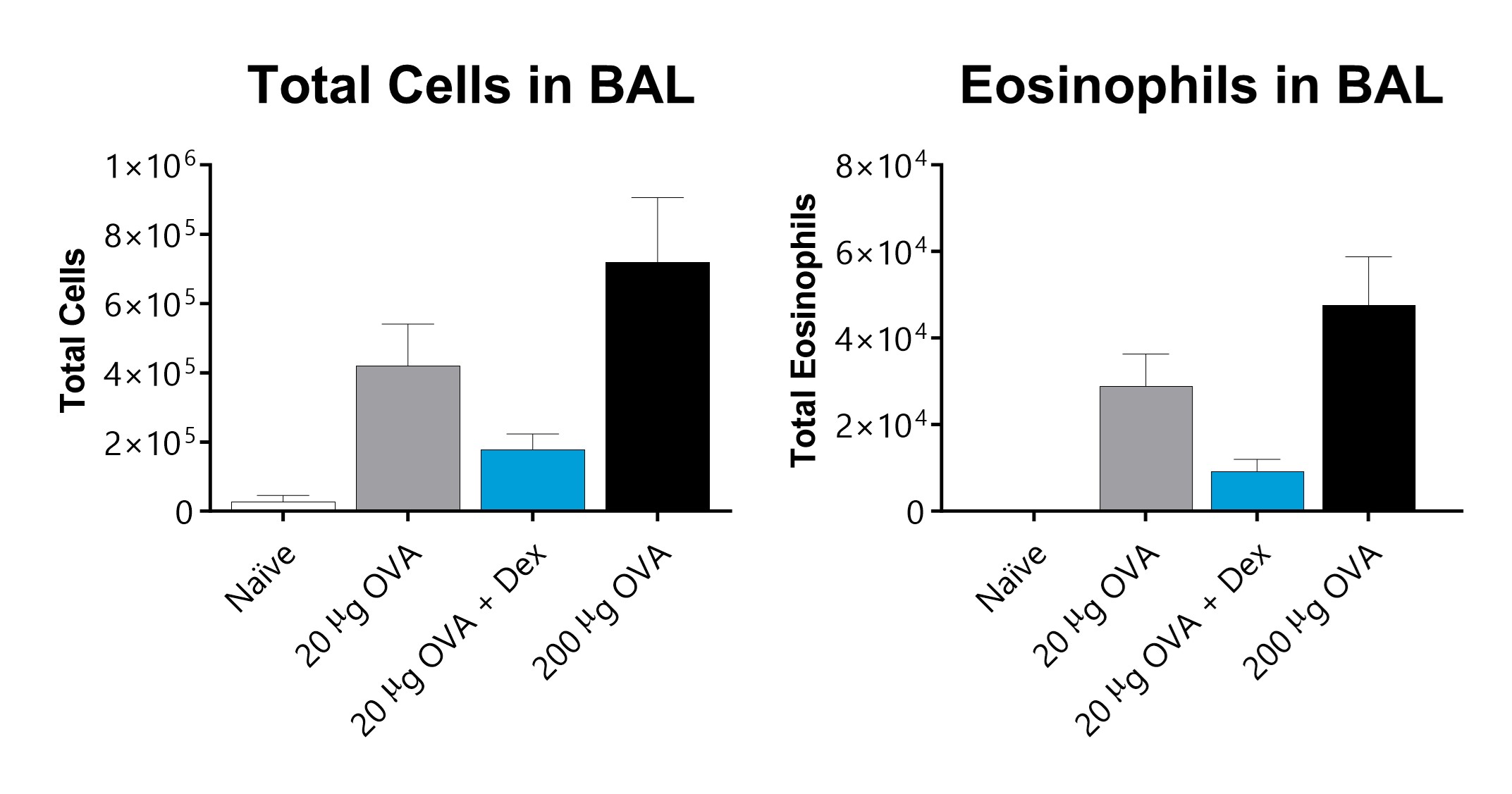
Total cells and eosinophils recover in broncho-alveolar lavage fluid on day 16 of an OVA-induced acute allergic asthma model. Mice demonstrate a dose responsive increase in total cells and eosinophils. Dexamethasone lower total inflammatory cell counts and eosinophils in the BAL fluid.
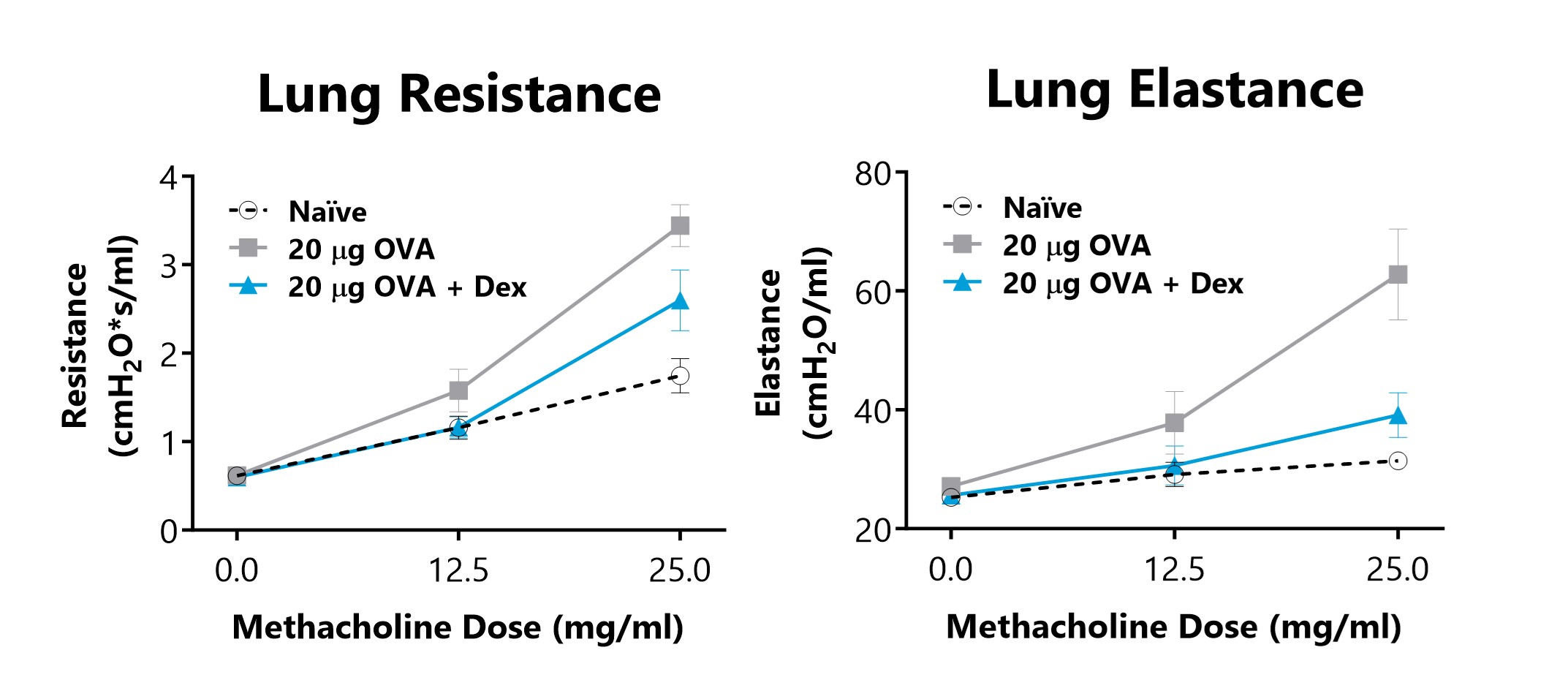
Lung Resistance and Elastance is measured on day 16 of an OVA-induced acute allergic asthma model, following exposure to increasing doses of methacholine. Animals that are sensitized and challenged with OVA displayed increased lung resistance and elastance parameters in comparison to Naïve animals. 3 mg/kg Dexamethasone treatment reduced airway hyperreactivity in diseased animals.
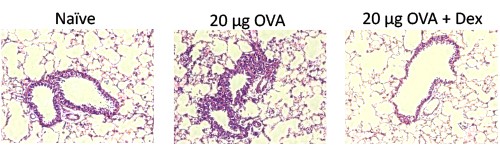
H&E-stained lung sections from naïve mice and mice from an OVA-induced acute allergic asthma model with and without 3 mg/kg dexamethasone treatment. The infiltration of mixed inflammatory cells observed in diseased animals is decreased in animals treated with 3 mg/kg dexamethasone.

Close

BioModels offers house dust mite (HDM) models of allergic asthma. An example of the acute HDM regimen would include sensitization on days 0 and 7 via the respiratory tract, challenge on day 14 with endpoints on day 15. Similar to the OVA model, the HDM models recapitulate many of the chronic asthma hallmarks seen in humans, however the HDM models utilize more clinically relevant allergens, as well as a more relevant route of sensitization, the respiratory tract.
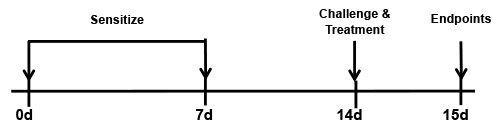
BALB/c mice are sensitized with an intranasal (IN) administration of HDM on days 0 and 7. Mice are then challenged with HDM intranasally on Day 14 and endpoints are assessed on day 15. Reference treatment animals receive 3 mg/kg dexamethasone 1 hour prior to the challenge.
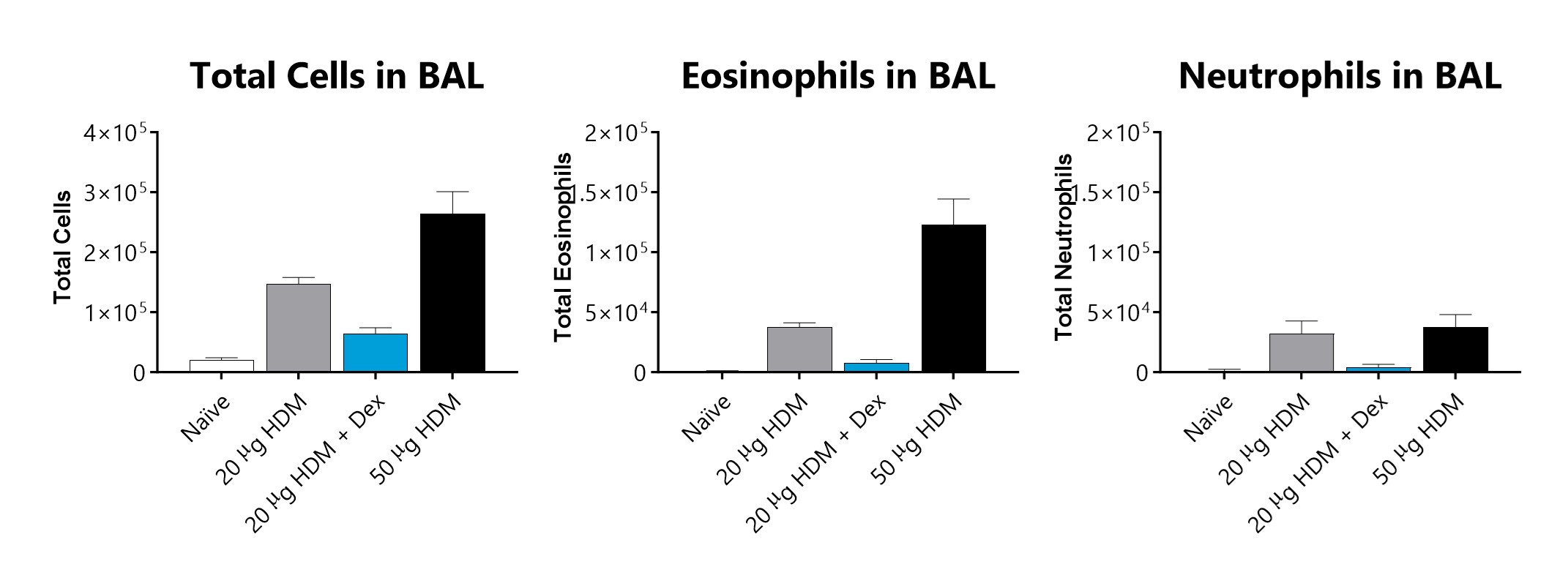
Total cells, eosinophils, and neutrophils recovered in broncho-alveolar lavage fluid on day 15 of an HDM-induced acute allergic asthma model. Sensitized and challenged mice demonstrate an increase in total cells, eosinophils, and neutrophils. 3 mg/kg dexamethasone treatment lowered total cells, eosinophils, and neutrophils in the BAL fluid.
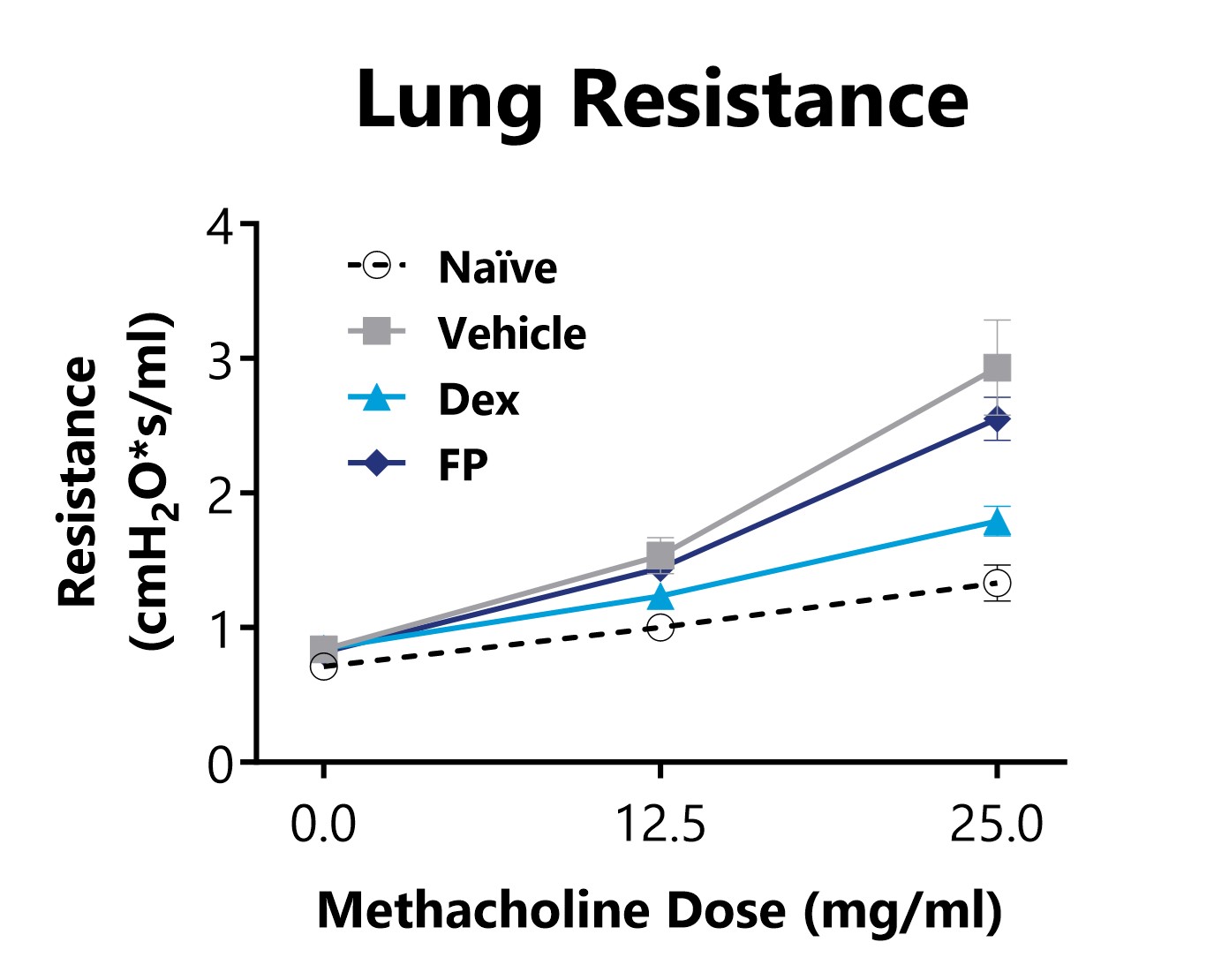
Lung Resistance is measured on day 15 of an HDM-induced acute allergic asthma model, following exposure to increasing doses of methacholine. Animals that are sensitized and challenged with HDM display increased lung resistance in comparison to Naïve animals. 3 mg/kg Dexamethasone or 1 mg/kg Fluticasone Propionate (FP) treatments reduced airway hyperreactivity in diseased animals.
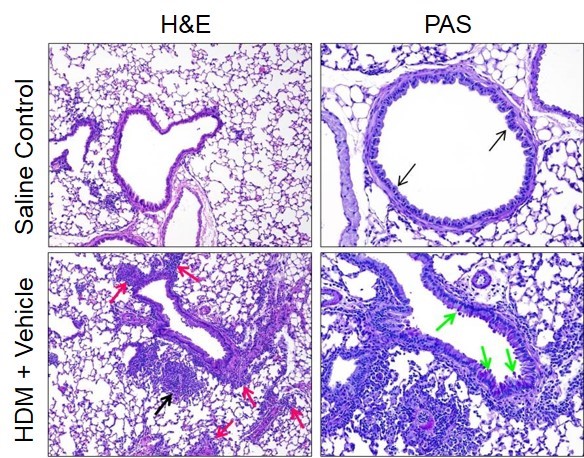
H&E- and PAS-stained lung sections from naïve mice and mice from an HDM-induced acute allergic asthma model on day 15. Infiltration of mixed inflammatory cells are observed in diseased animals in comparison to Naïve animals. Increases in multifocal inflammation within alveolar spaces (black arrow) and surrounding vessels (red arrows) are observed in lungs of diseased animals. Larger bronchioles lined with moderate numbers of PAS-positive goblet cells (green arrows) can be seen in lungs of diseased animals whereas saline control lungs had PAS-negative cells (black arrows).
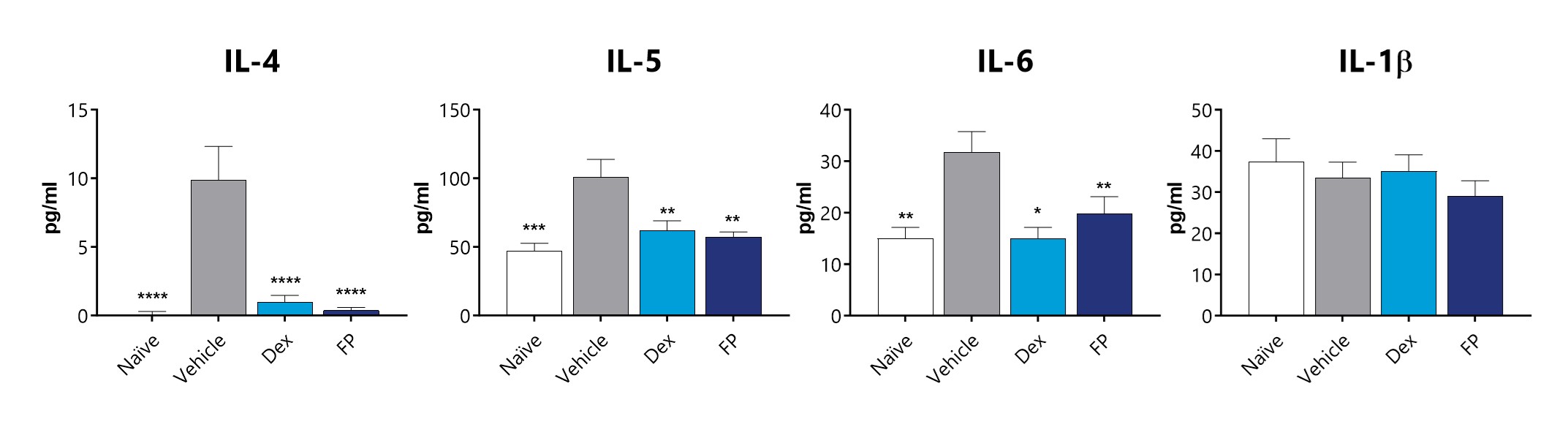
Significant increases in Th2 inflammatory mediators (IL-4, IL-5, and IL-6) are observed in BAL fluid of diseased animals, though changes in IL-1β, a Th1 cytokine are not observed. Levels of IL-4, IL-5, and IL-6 in BAL fluid are significantly lower in animals treated with either 3 mg/kg Dexamethasone (Dex) or 1 mg/kg Fluticasone Propionate (FP). (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 compared to the vehicle-control)
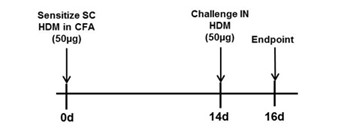
BALB/c mice are sensitized with a subcutaneous (SC) administration of 50 µg HDM in CFA on day 0. Mice are then challenged with 50 µg HDM intranasally on Day 14 and endpoints are assessed on day 16. Reference compound and test article treatments are generally administered on or around days 13-16.
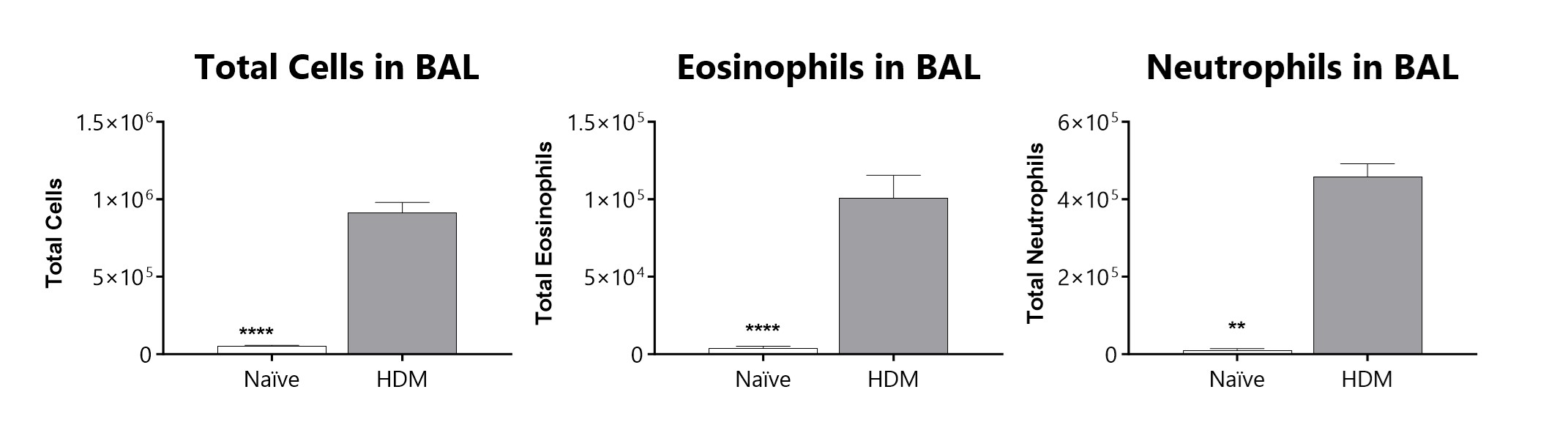
Total cells, eosinophils, and neutrophils recovered in broncho-alveolar lavage fluid on day 16 of an HDM-induced severe asthma model. Sensitized and challenged mice demonstrate a significant increase in total inflammatory cells, eosinophils, and neutrophils in BAL fluid. (*p<0.05; ****p<0.0001 compared to the vehicle-control)
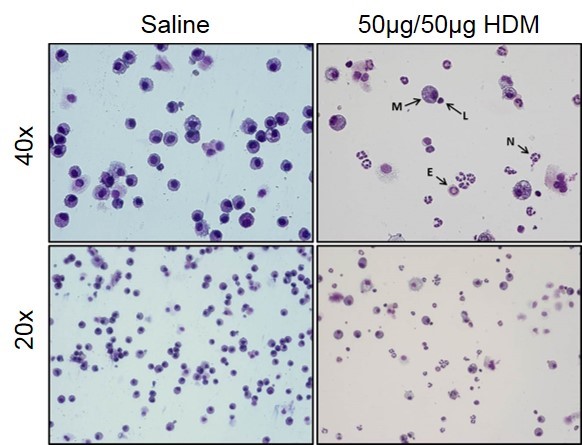
Images from control (saline) and severe asthma model (50µg/50µg HDM) BAL fluid. Macrophages (M), Lymphocytes (L), Neutrophils (N), and Eosinophils (E) can all be observed in the BAL fluid from mice in the severe asthma model.
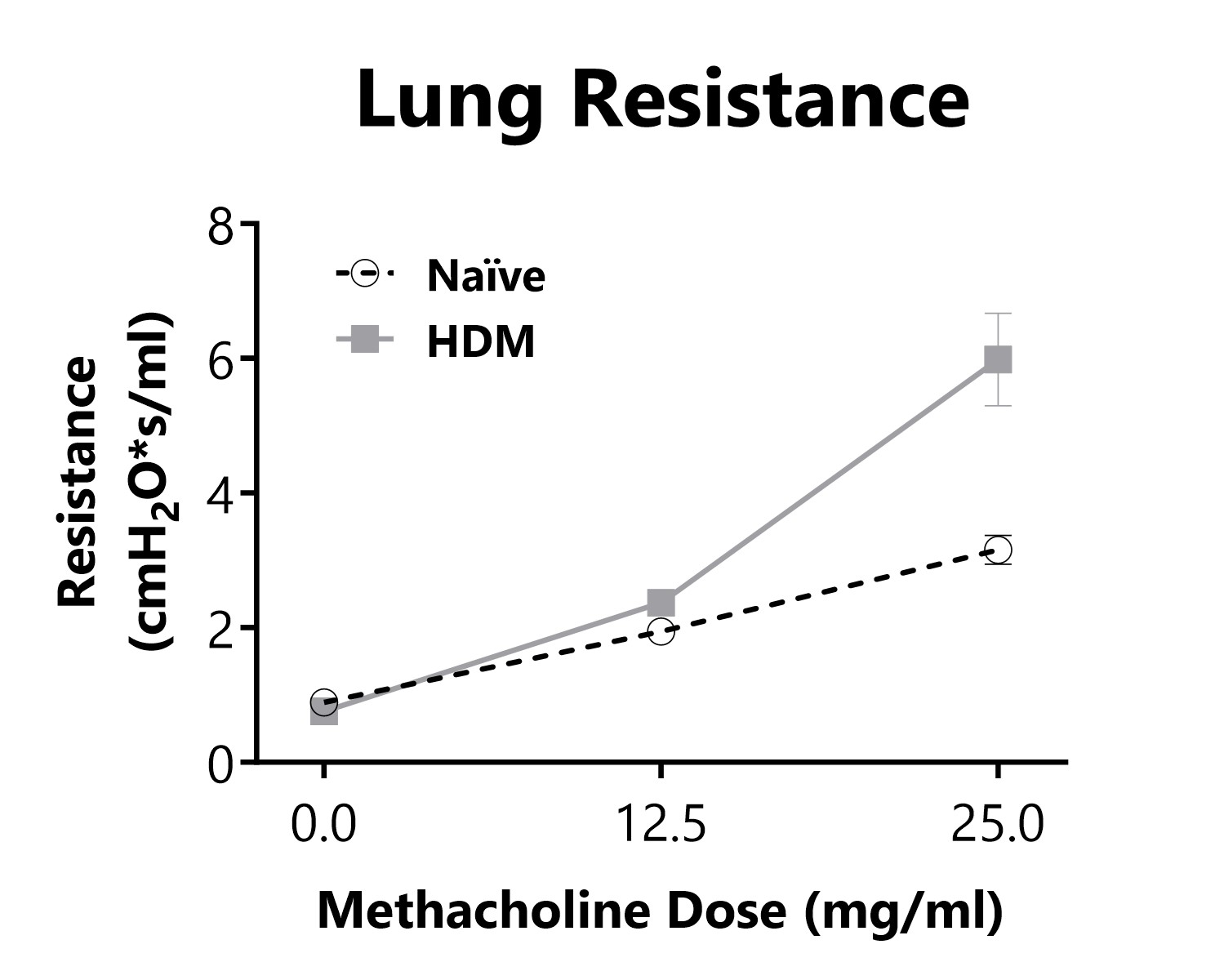
Lung Resistance is measured on day 16 of an HDM-induced severe asthma model, following exposure to increasing doses of methacholine. Animals that are sensitized and challenged with HDM display increased lung resistance in comparison to Naïve animals.
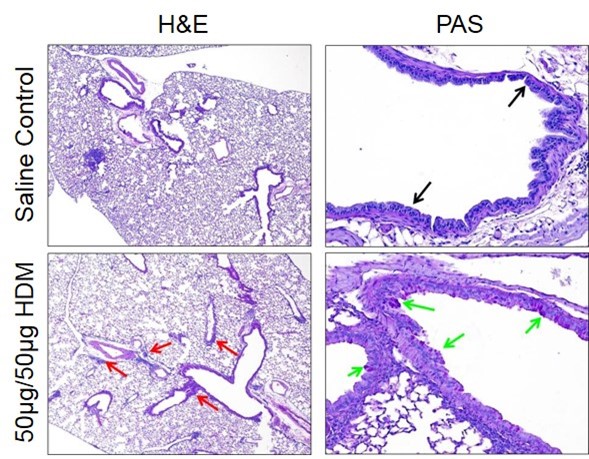
H&E- and PAS-stained lung sections from naïve mice and mice from an HDM-induced severe asthma model on day 16. An increased presence of perivascular and peribronchiolar inflammation (red arrows) is observed in diseased animals. Larger bronchioles of diseased animals are lined with moderate numbers of PAS-positive goblet cells (green arrows) whereas saline control lungs had PAS-negative cells (black arrows).
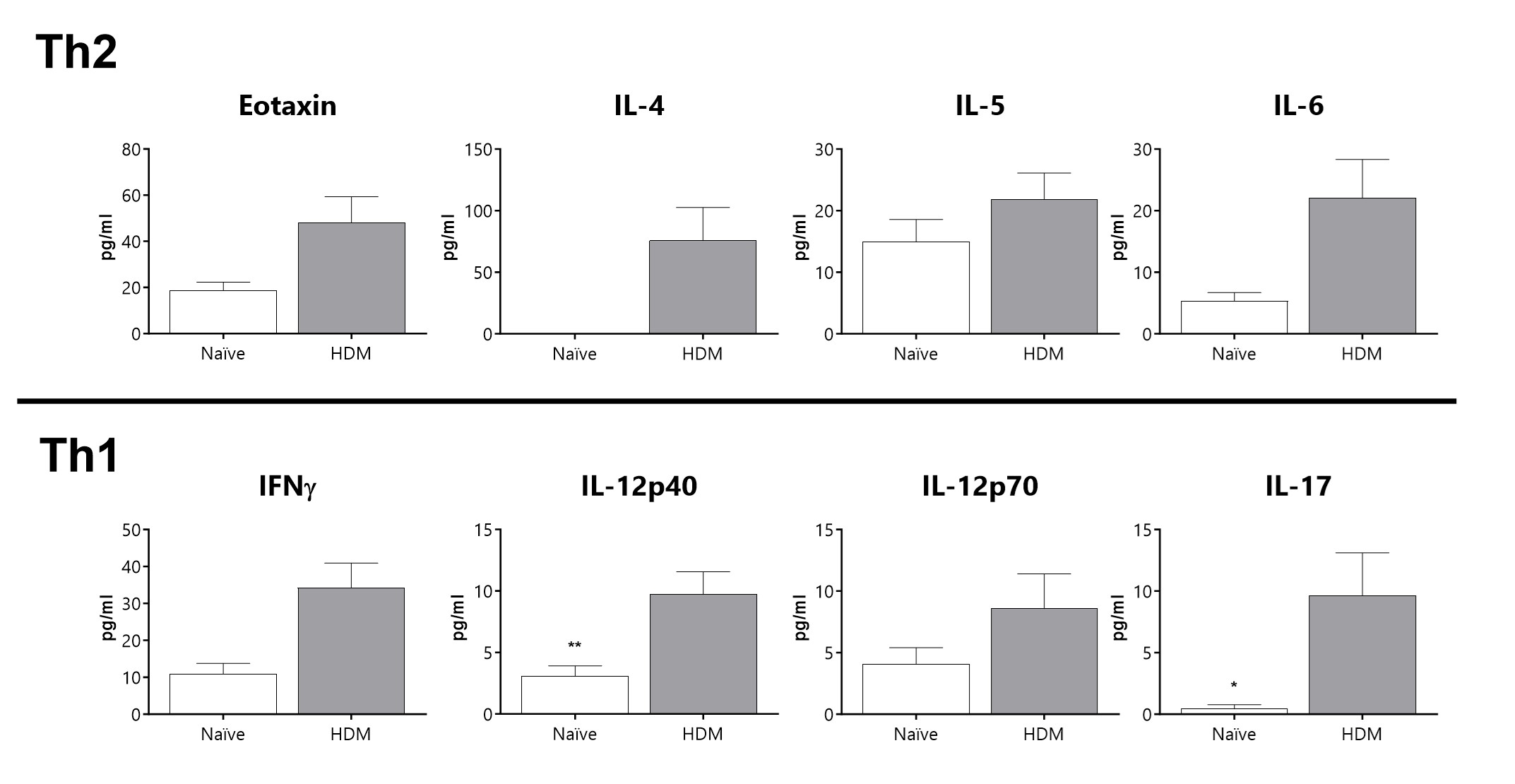
A mixed Th1/Th2 cytokine inflammatory profile in BAL fluid of diseased animals is observed in a severe asthma phenotype.

Close

Various allergen sensitization models are used in the literature to induce Eosinophilic Esophagitis (EoE), a chronic, type 2 inflammatory condition of the esophagus. BioModels offers a model of Eosinophilic Esophagitis in which Balb/c mice are sensitized with ovalbumin early in the study and challenged with ovalbumin via an alternate route weeks after the study initiation. The animals are monitored daily and while no overt signs of disease are typically observed (i.e. body weight loss), increased eosinophil cell infiltration is observed by histopathological evaluation of the esophageal tissue following OVA sensitization and challenge. Analysis of local and systemic cytokine protein levels may also be of interest when evaluating efficacy of a potential therapeutic. Please contact us to discuss customizing the model to fit your study goals.
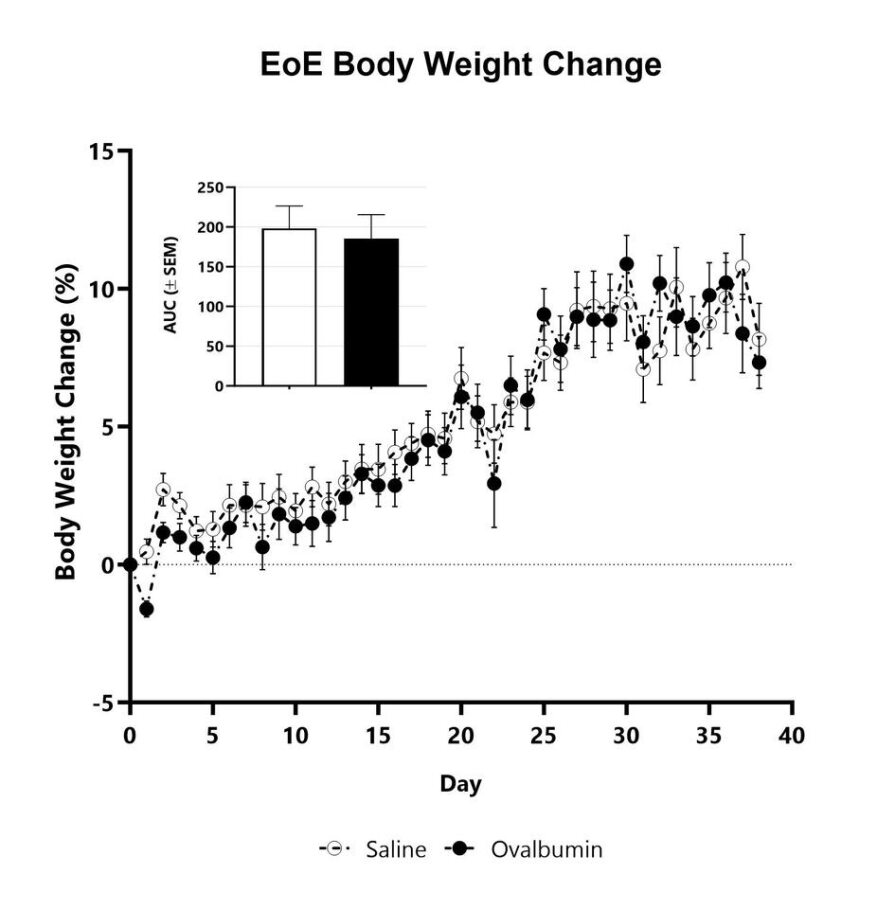
Animals with Eosinophilic Esophagitis are weighed daily, and body weight change as compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset.
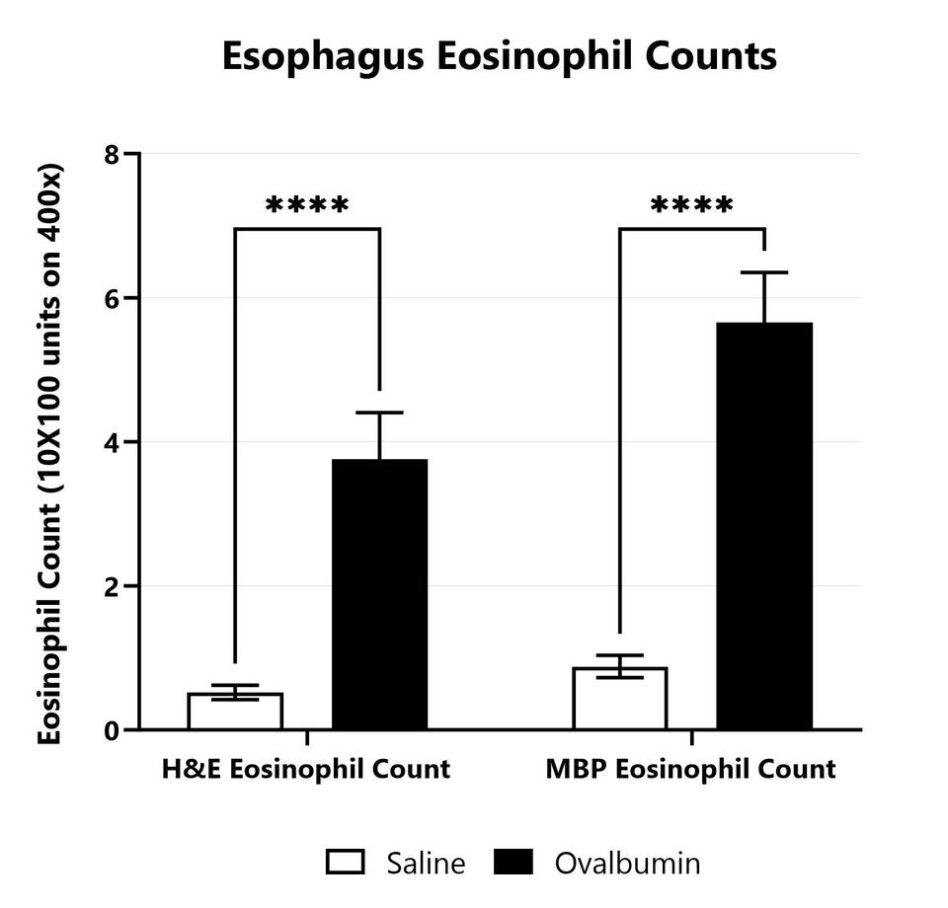
Esophagus tissue from saline treated and OVA sensitized Balb/c mice is collected upon euthanasia. Sections are prepared from FFPE tissue. Five high-power fields of any area of esophagus containing eosinophils (on H&E or MBP stains) are counted using the 63X objective and an ocular micrometer defining a rectangle with 10 X 100 units (15.7 µm x 157 um; 2,464.9 µm2). Scores are assigned for each stain after determining the mean of the five representative fields.

Close

BioModels offers a variety of food allergy models which can be designed specific to your hypothesis, with customized allergen and sensitization options available. Food allergy is a serious health concern that can result in local inflammation, hives, as well as anaphylaxis. Animals may be sensitized by multiple exposures to an allergen over the course of the study, with or without adjuvant, followed by challenge. Allergic phenotypes may include anaphylaxis (hypothermia; measured via implanted temperature probes), changes in biomarkers (antigen-specific immunoglobulin), GI inflammation (assessed via endoscopy), changes in animal behavior, or changes in various immunological parameters

Close
Study Models

The Azoxymethane/Dextran sodium sulfate (AOM/DSS) model of inflammation associated polyposis is a routinely utilized model that accurately recapitulates many features of human disease. Animal exposure to AOM, a carcinogen, results in a somatic inactivating mutation in a Wnt pathway kinase, leading to the generation of spontaneous cancerous colorectal polyps. The addition of DSS damages the mucosal layer, with concomitant inflammation, exacerbating the AOM-induced phenotypes. Weight loss with resolution is observed following the DSS-administration period. Polyps begin to develop approximately one week later and grow progressively, with some ultimately forming adenomas or adenocarcinomas.
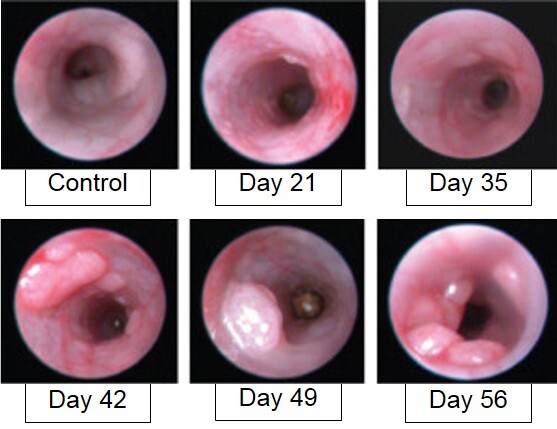
AOM/DSS-induced animals are assessed for colon inflammation and polyp development by video endoscopy.

Close

The Sclerodermatous Chronic GVHD model is a minor MHC mismatched model commonly used to study pathogenesis of fibrosis in cGVHD, and frequently used in drug discovery and development. Sclerodermatous Chronic GVHD model pathology involves immune cell mediated release of Th2 cytokines and fibrosis pathways. Recipient C57Bl/6 mice are pre-conditioned with a lethal dose of total body irradiation (TBI) prior to the adoptive transfer of bone marrow that is supplemented with splenocytes harvested from a minor MHC-mismatched donor mouse (LP/J). Following irradiation and transplant, the recipient mice show transient weight loss that typically recovers by Day 21 to 28. After this 3-4 week period, animals develop a progressively worsening GVHD that is characterized by weight loss, increasing GVHD score, and development of sclerodermatous skin lesions. Test article responses can be compared to the effects of Ruxolitinib.

Recipient mice are pre-conditioned and transplanted with donor-derived bone marrow cells supplemented with splenocytes on Day 0. Animals are weighed daily, and body weight change as compared to Day 0 is calculated (top). To normalize for survivor bias, percent weight change with death weight carried forward is calculated (bottom). The AUC is calculated to compare treatment groups and is shown in the inset. (****: p<0.0001; compared to GVHD-Vehicle group)

GVHD mice are assessed daily for weight loss, posture, activity, fur texture, and skin integrity phenotypes using our standard GVHD scoring scale. Each phenotype is scored and used to calculate the composite GVHD score (top). To normalize for survivor bias, GVHD score with death score carried forward is calculated (bottom). The AUC is calculated to compare treatment groups and is shown in the inset. (*: p<0.05; **: p<0.01 compared to GVHD-Vehicle group)

GVHD mice are assessed daily for weight loss, posture, activity, fur texture, and skin integrity phenotypes using our Sclerodermatous Chronic GVHD scale. Each phenotype is scored and used to calculate the composite Sclerodermatous Chronic GVHD score (top). To normalize for survivor bias, Sclerodermatous Chronic GVHD score with death score carried forward is calculated (bottom). The AUC is calculated to compare treatment groups and is shown in the inset. (*: p<0.05; **: p<0.01 compared to GVHD-Vehicle group)

Animals are assessed for survival and moribundity on a daily basis. (*: p<0.05 compared to GVHD-Vehicle group)

Progression free survival is tracked for the duration of the study and percent progression free survival is calculated for the standard GVHD scale (top) and the Sclerodermatous Chronic GVHD scale (bottom).


Close

The most common form of inflammatory arthritis, gout, is the result of hyperuricemia that leads to monosodium urate crystal (MSU) formation in the synovium and joint. At BioModels, we utilize an intra-articular injection of MSU crystals in both rats and mice to induce an acute local inflammation characterized by swelling of the affected joint. Gout assessment using a validated 5-point scoring scale as well as ankle measurements (2 planes) are evaluated at multiple points during the in-life phase of the study. Additional assessments may include static weight bearing, plantar testing, and pain assessments. At the conclusion of the study, tissues are collected for assessment of inflammatory mediators and other biomarkers of interest. Colchicine is commonly used as a comparator compound.
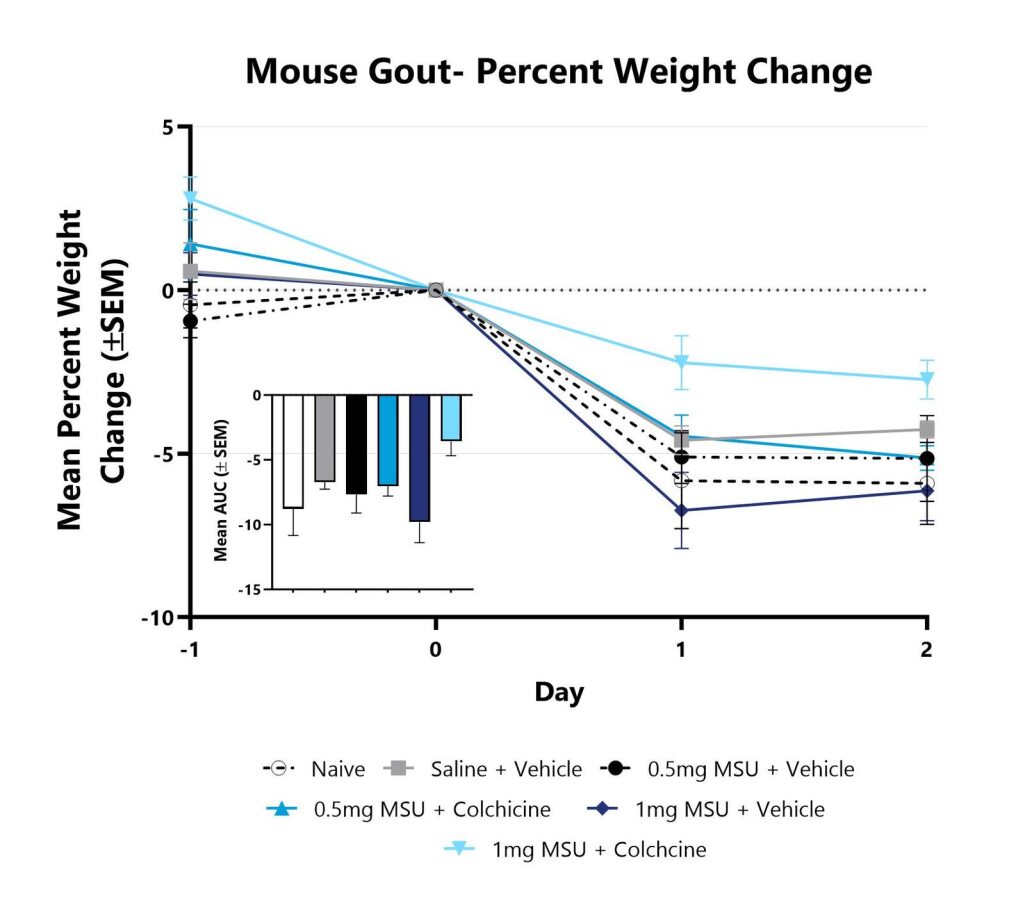
Animals are weighed daily, and body weight changes as compared to Day 0 are calculated. The AUC is calculated to compare treatment arms and is shown in the inset.
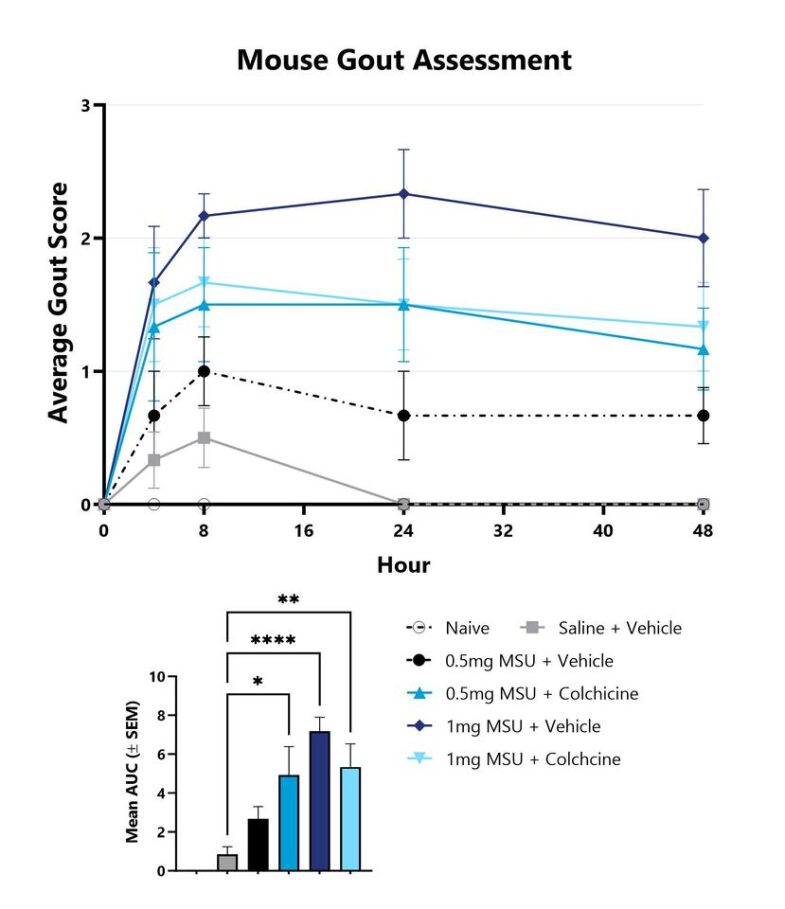
Mice with MSU-induced gout are assessed for clinical gout development over the course of the study. The affected ankle of each animal is scored on a validated scale of 0-4. The group average score is displayed. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05; **p<0.01; ****p<0.001 compared to vehicle control).
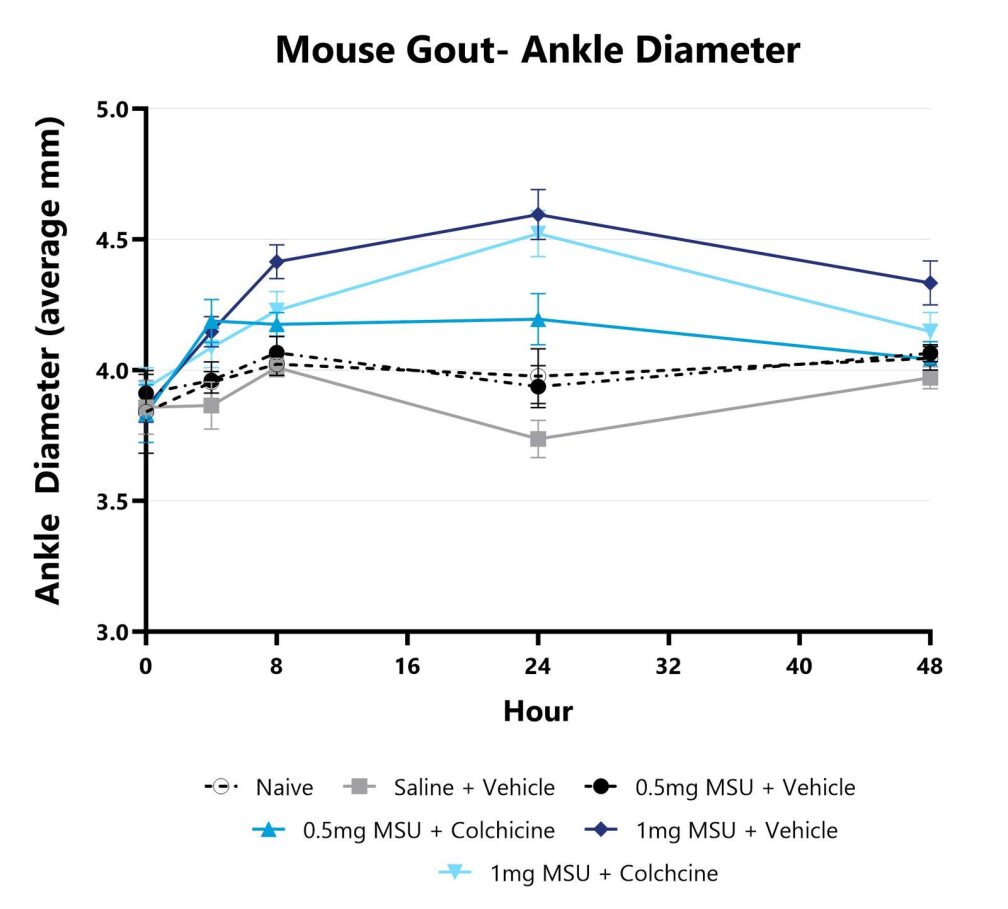
Ankle diameter is assessed over the course of the study by measurement with a digital caliper. The affected ankle is measured in 2 planes and the average for each animal is calculated.
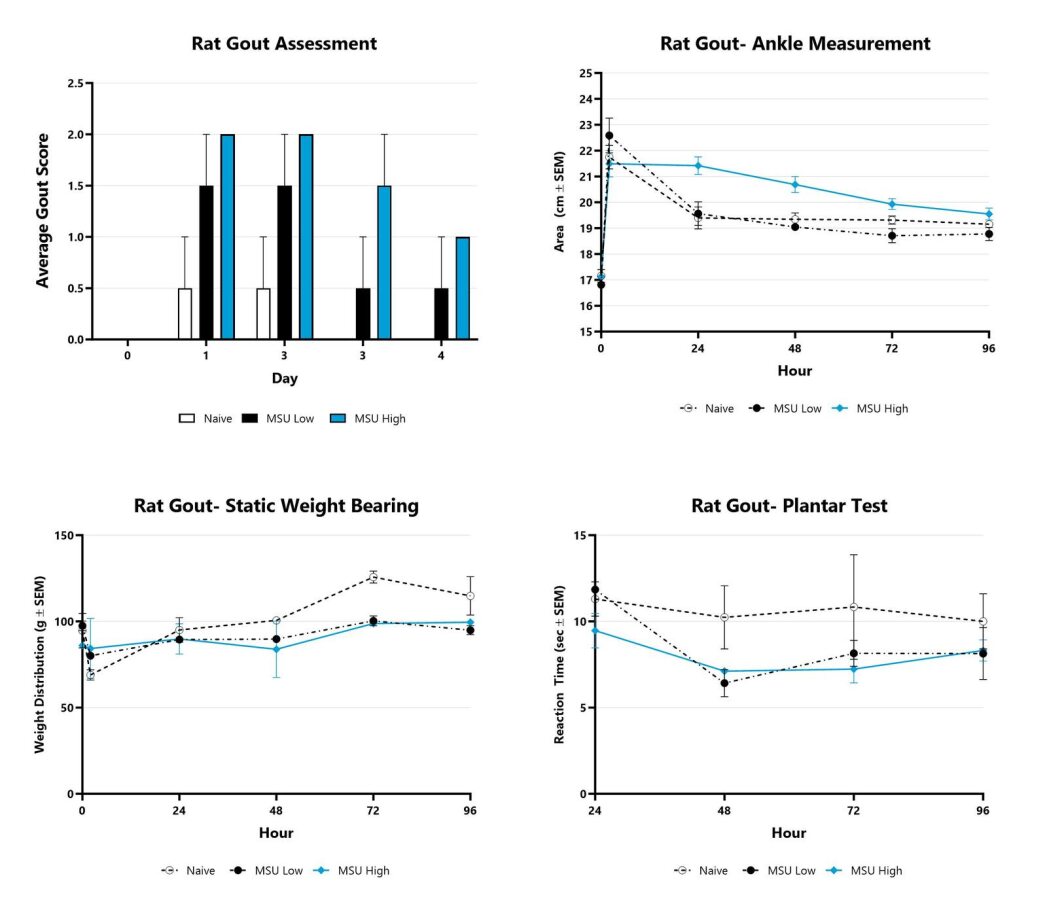
Rats with MSU-induced gout are assessed for clinical gout development over the course of the study. The affected ankle of each animal is scored on a validated scale of 0-4. The group average score is displayed. The ankle area is assessed over the course of the study by measurement with a digital caliper. The affected ankle is measured in 2 planes and the area for each animal is calculated. Static weight bearing measurements and reaction time (Plantar Test) are assessed at multiple times during the study.

Close

Acute pancreatitis is one of the most common diseases in gastroenterology. The CAE-induced model of pancreatitis in rodents is widely used and one of the best characterized models. Administration of repeated doses of CAE results in an increase in pancreatic digestive enzyme levels leading to an increase in cytokine production and subsequent inflammatory cell infiltration. In addition, reactive oxygen species (ROS) production leads to oxidative stress and cell apoptosis, which is a major pathogenic factor in acute pancreatitis. At BioModels, animals are administered multiple doses of CAE spaced one hour apart. Five hours after induction, the animals are anesthetized, and pancreatic blood flow is measured using a laser Doppler system. The animals are euthanized, and tissues collected for downstream analyses. Primary endpoint analyses include assessment of cell death (apoptosis or necrosis), inflammation, edema, and a reduction in pancreatic blood flow. Many variations of the CAE-induced pancreatitis model exist, contact us to discuss customizing the model to fit your needs.
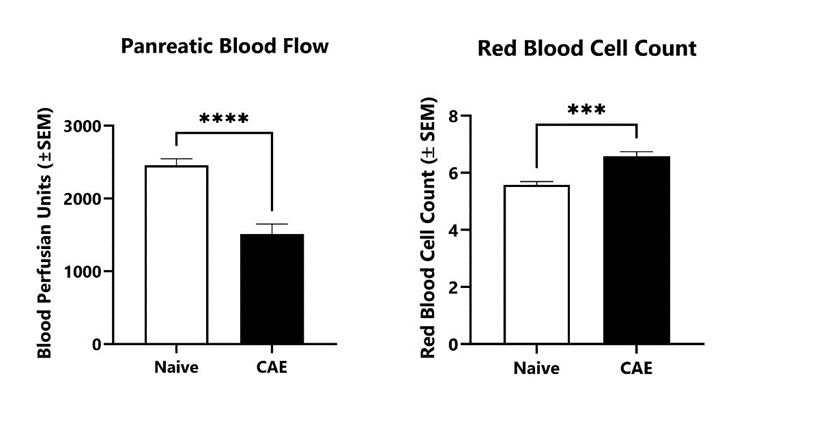
Following 5 injections of caerulein, pancreatic blood flow and red blood cells counts are determined in the acute pancreatitis model using a laser Doppler system and complete blood count (CBC), respectively. (***p<0.001; ****p<0.0001 compared to Naive control).

Close

Glomerulonephritis, a group of diseases that result in inflammation and subsequent injury to the glomeruli in the kidneys, can be acute and self-resolving or chronic leading to possible kidney failure. In some patients, pathogenic autoimmune antibodies directed towards renal antigens such as glomerular basement membrane bind their target, activating Fcγ receptors and forming inflammatory immune complexes. At BioModels, anti-GBM nephritis is induced by passive transfer of a bolus of nephrotoxic sheep antisera to mice or rats. Tissue samples are collected from one to six days following serum transfer to assess kidney function and cytokine production. Histopathological analyses of the kidneys provide a more detailed look at specific kidney structure such as tubular, interstitial, and glomerular changes.
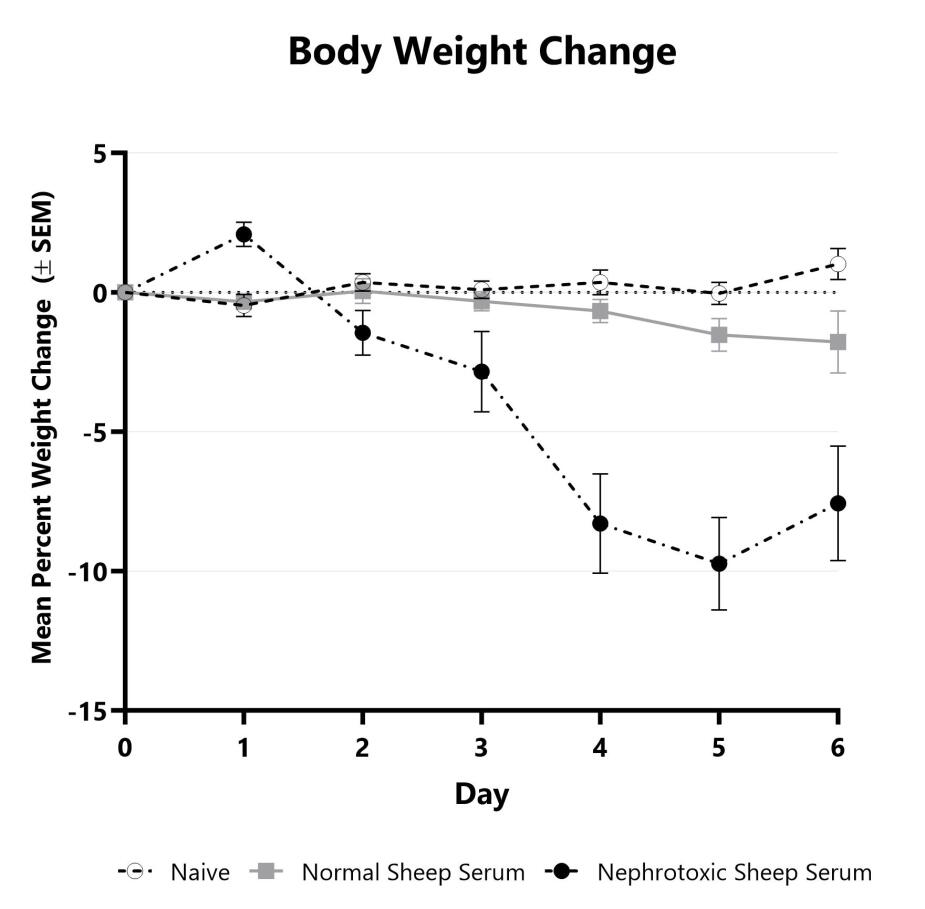
C57Bl/6 mice are weighed daily, and body weight changes as compared to Day 0 are calculated. The AUC is calculated to compare treatment arms and is shown in the inset.
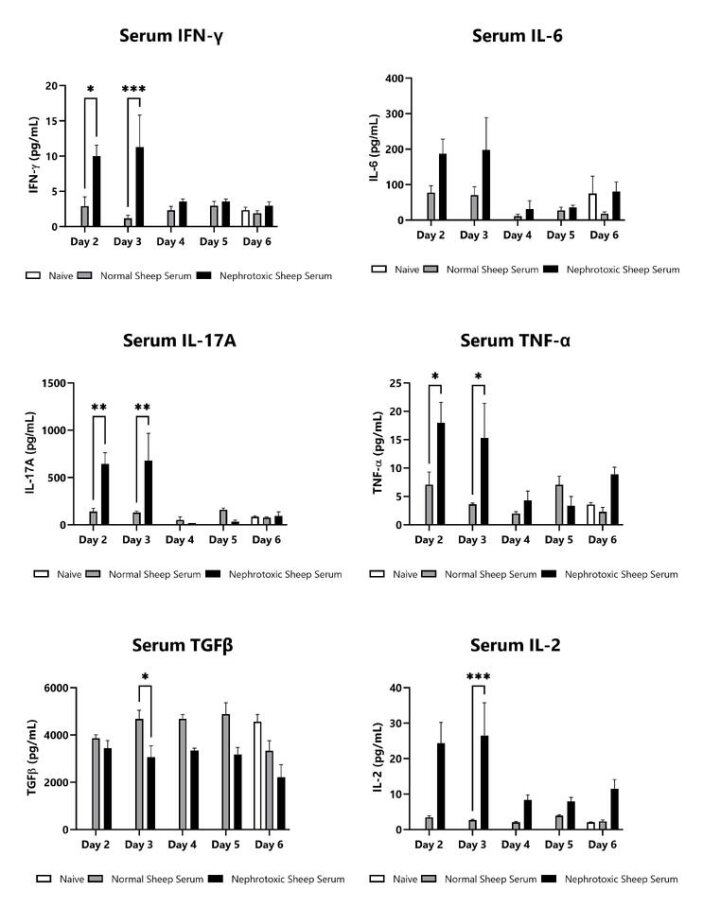
Blood samples are collected on Days 2, 3, 4, 5, and 6 following anti-GMB nephrotoxic serum injection. Blood is processed for serum and cytokines IFN-γ, IL-6, IL-17A, TNF-α, and IL-2 are assessed via multiplex and ELISA (TGFβ). Additional cytokines are available. (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
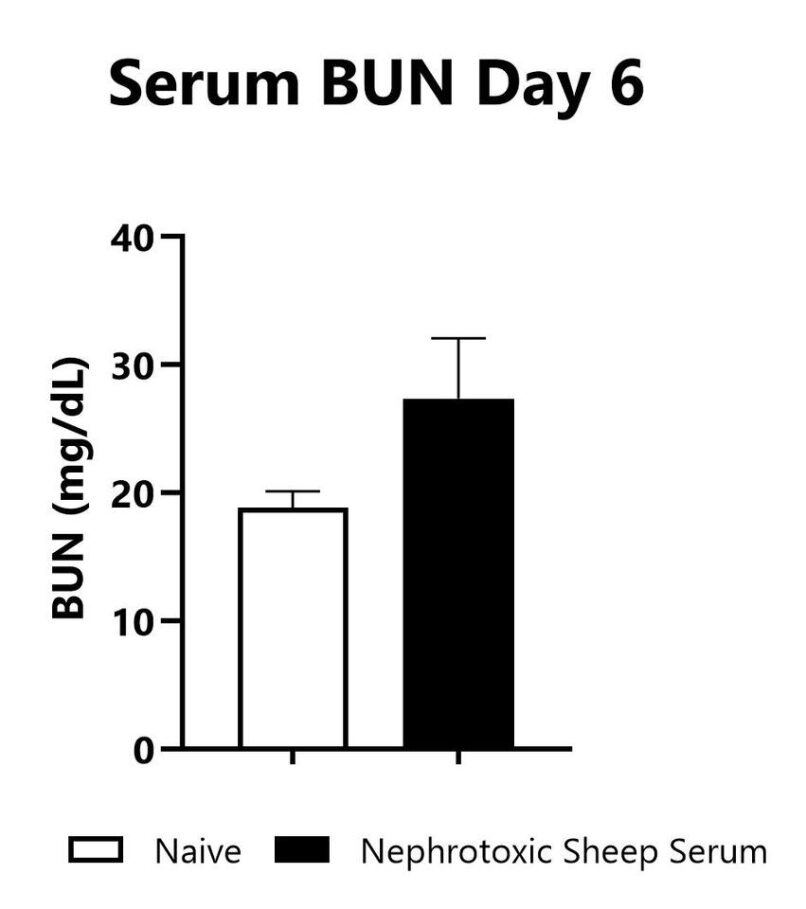
Blood samples are collected on Day 6 following anti-GMB nephrotoxic serum injection. Blood is processed for serum and chemistries are analyzed using a VetScan Chemistry Analyzer. Serum BUN levels in naïve mice and in mice that were administered nephrotoxic sheep serum are shown.
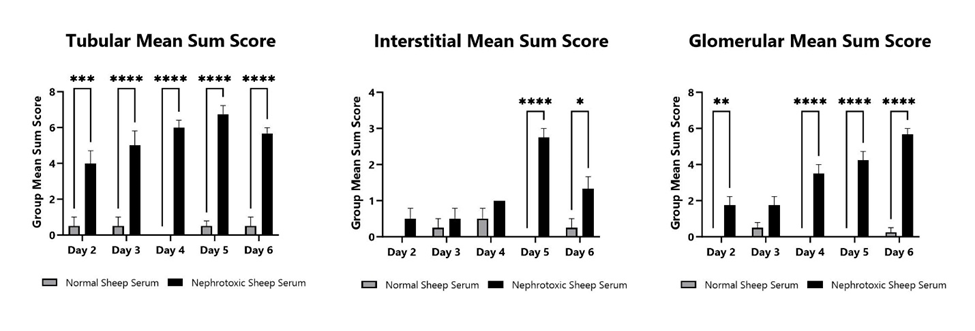
Kidneys are collected on Days 2, 3, 4, 5, and 6 following anti-GMB nephrotoxic serum injection. Kidneys are fixed, paraffin embedded, and stained with H&E and PAS (periodic acid-Schiff) stain. Tubular, interstitial, and glomerular changes are semi-quantitatively analyzed by a board-certified Veterinary Pathologist and mean sum scores are shown.

Close