- Research Services
- Capabilities
- About Us
- Resources
- Contact Us
 Pulmonary Fibrosis
Pulmonary Fibrosis Dermal Fibrosis
Dermal Fibrosis Radiation Fibrosis
Radiation Fibrosis Liver Fibrosis
Liver Fibrosis Additional Fibrosis Disease Models
Additional Fibrosis Disease Models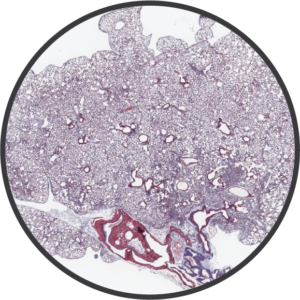 Fibrosis
FibrosisBioModels has extensive experience with a variety of animal models that capture critical mechanisms of fibrosis.
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established pulmonary fibrosis models. Pulmonary fibrosis is a chronic/progressive lung disease that presents with difficulty breathing due to thickened and scarred lung tissue. BioModels’ pulmonary fibrosis models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis. The BioModels team is experienced with numerous preclinical pulmonary models, including bleomycin- and radiation-induced in vivo models and an in vitro model of fibrosis.
Experimental endpoints for these models include:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established dermal fibrosis models. Dermal fibrosis presents as thickening of the skin that occurs due to the deposition of excess collagen and connective tissue and is associated with diseases such as scleroderma (systemic sclerosis) and chronic graft-versus-host disease (cGvHD). BioModels’ dermal fibrosis models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis. The BioModels team is experienced with numerous preclinical dermal fibrosis models, including bleomycin- and radiation- induced models.
Experimental endpoints for these models include:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established radiation-induced fibrosis models. Radiation-induced fibrosis often manifests in various tissues of cancer patients receiving radiation therapy, which can lead to organ dysfunction and decreased quality of life. BioModels’ radiation-induced fibrosis models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis. The BioModels team is experienced with numerous preclinical radiation-induced fibrosis models, utilizing single hit and fractionated radiation to promote fibrotic disease.
Experimental endpoints for these models include:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established liver fibrosis models. Liver fibrosis is common outcome of a number of chronic liver diseases and if left untreated can lead to liver failure, liver cancer, and death. BioModels’ liver fibrosis models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis. The BioModels team is experienced with numerous preclinical liver fibrosis models, including chronic carbon tetrachloride- and diet-induced in vivo models.
Experimental endpoints for these models include:
Assess your experimental materials for efficacy and mechanism of action in BioModels’ established fibrosis models. Fibrosis is a chronic/progressive disease that presents with organ dysfunction and can lead to death. BioModels’ fibrosis models capture a spectrum of disease mechanisms, allowing you to select the model most relevant for your hypothesis. The BioModels team is experienced with numerous fibrosis models including renal fibrosis and systemic sclerosis.
Experimental endpoints for these models include:
Study Models

The intratracheal (IT) bleomycin-induced pulmonary fibrosis model is the standard model of human lung fibrosis. After being anesthetized, animals have a cannula inserted into the trachea and down into the lungs and then bleomycin is slowly infused into the lungs. Animals are evaluated daily for body weight and respiratory status. After animals are sacrificed, lung weight, collagen content and fibrosis scores are determined.
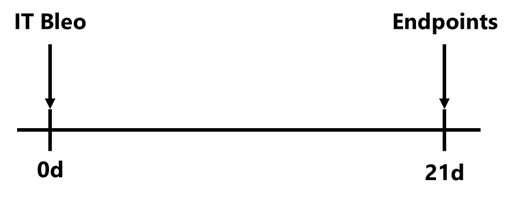
C57Bl/6 mice are administered Bleomycin once on Day 0 by intratracheal route. Endpoints are assessed on Day 21.
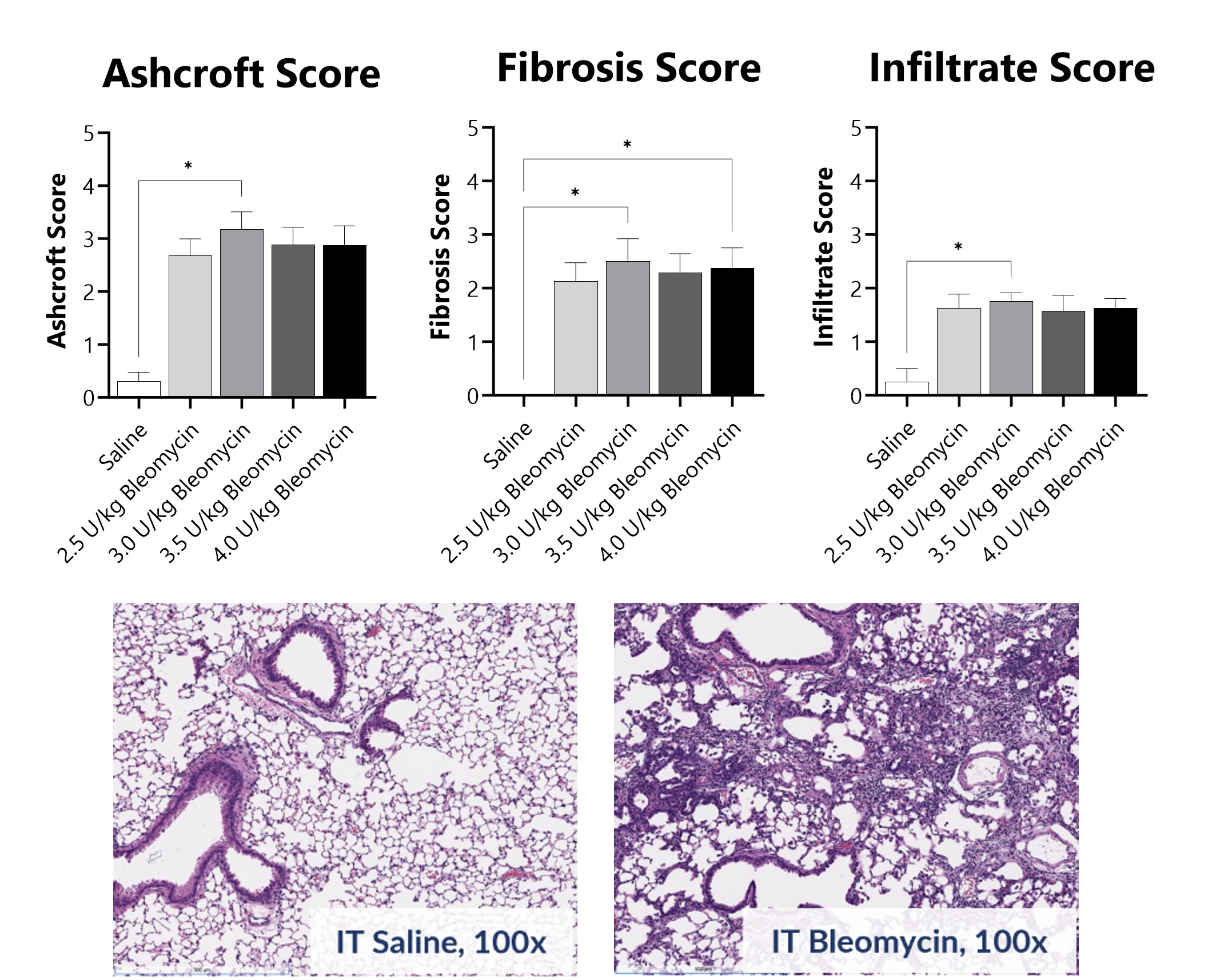
Pathology assessment of Day 21 lungs from IT-Bleomycin administered animals. Ashcroft score was determined in H&E-stained tissue using a modified Ashcroft Scale graded from 0-8. Fibrosis score was determined in Masson’s Trichrome-stained tissue using a 0-5 semi-quantitative scoring scale of area staining positive for collagen. Infiltrate score was determined in H&E-stained tissue using a 0-5 semi-quantitative scoring scale based on severity of mononuclear cell infiltration. Images are from animals receiving saline or 3.0 U/kg Bleomycin. Histopathology, immunohistochemistry, and analysis were performed by Dallas Tissue Research. (*p<0.05 compared to the saline-control)
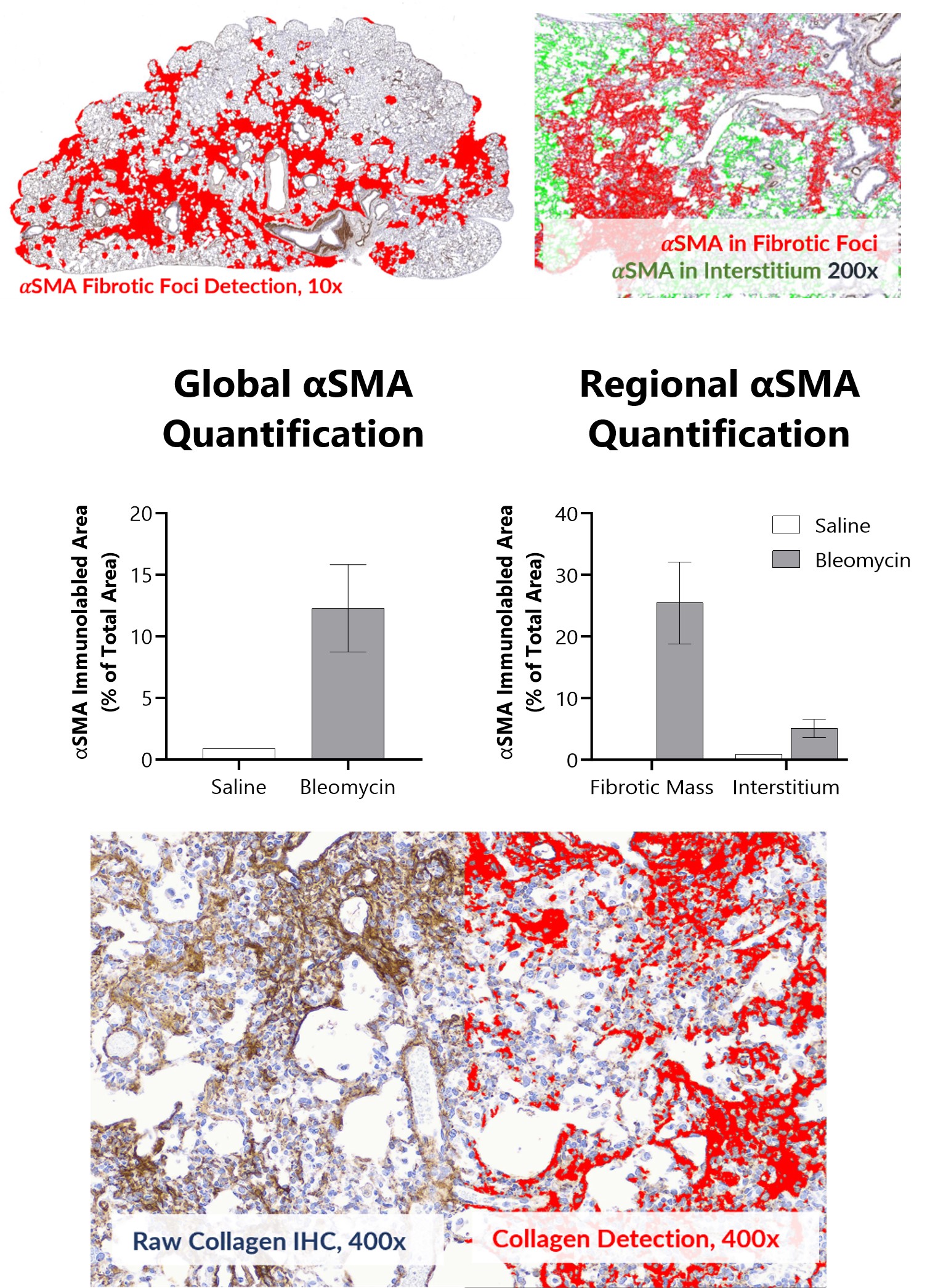
Alpha smooth muscle actin (αSMA) and Collagen I + III Immunohistochemistry in Day 21 lungs from IT-Bleomycin administered animals. Whole slide images were used for global and regional αSMA quantification using a deep learning algorithm. Images are from animals administered either saline or 3.0 U/kg Bleomycin. Histopathology, immunohistochemistry, and analysis were performed by Dallas Tissue Research.
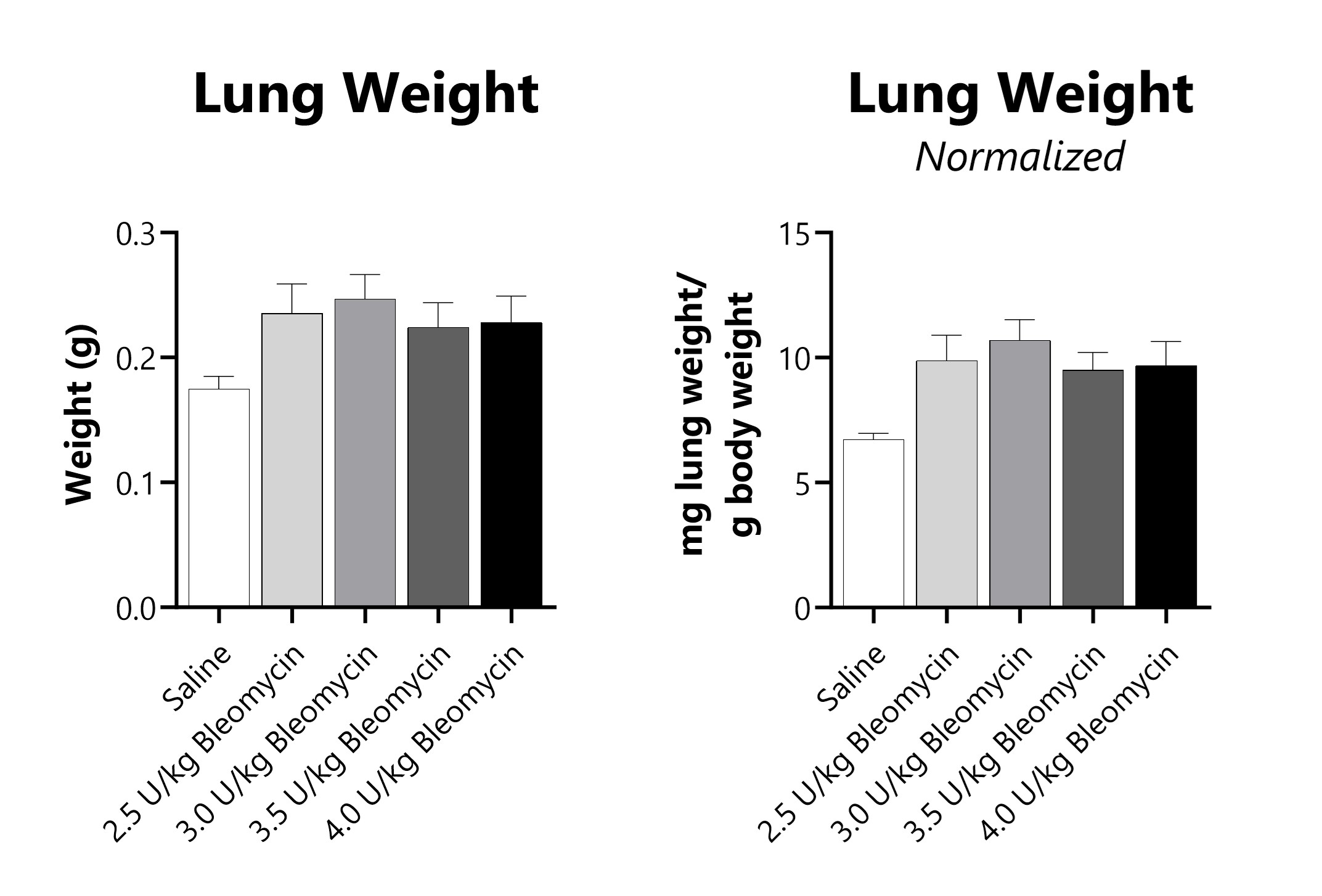
Wet lung weight and wet lung weight normalized to Day 21 body weight of Day 21 lungs from IT-Bleomycin administered animals.
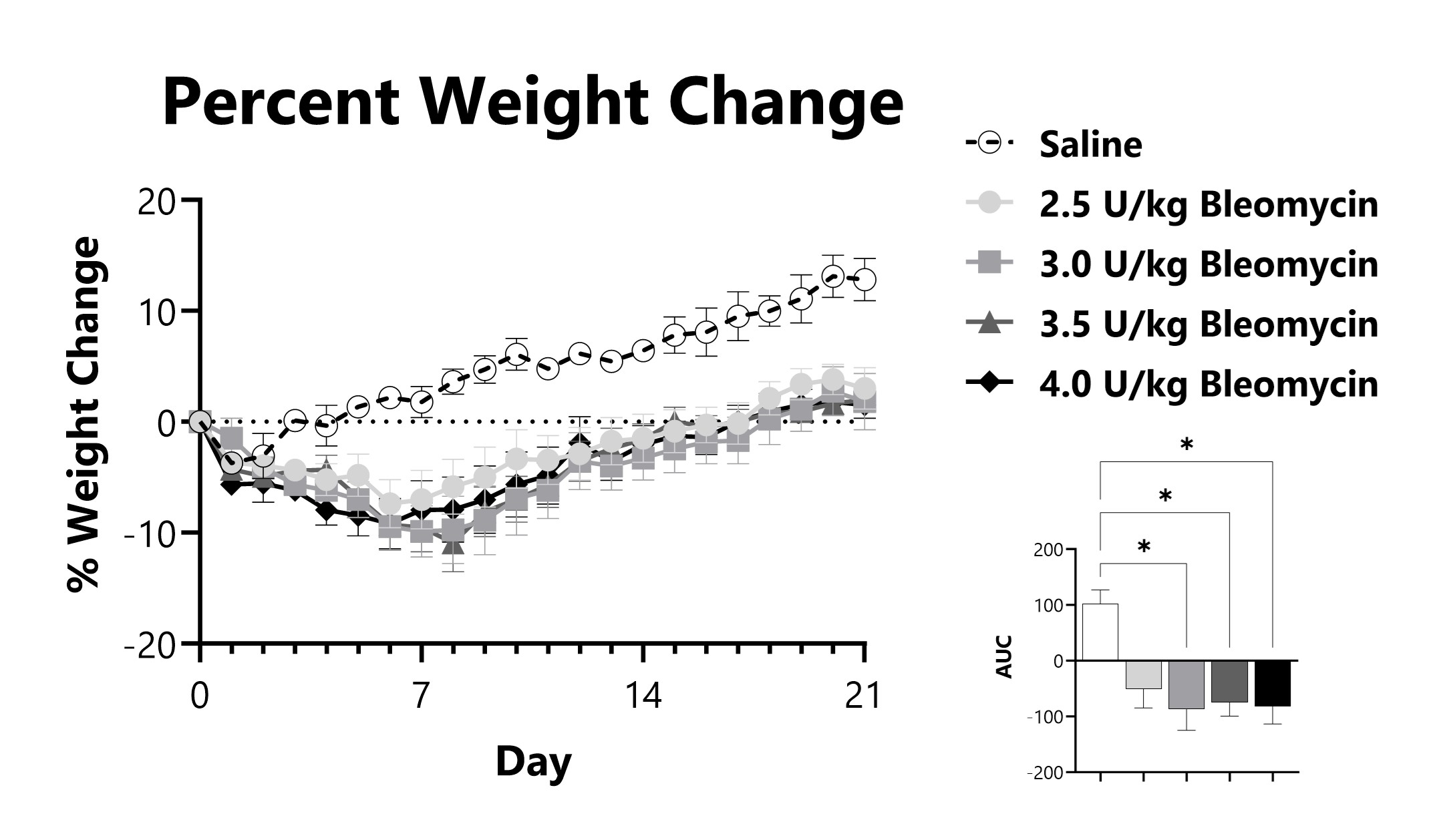
IT-Bleomycin administered animals are weighed daily, and body weight compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (*p<0.05 compared to the saline-control)
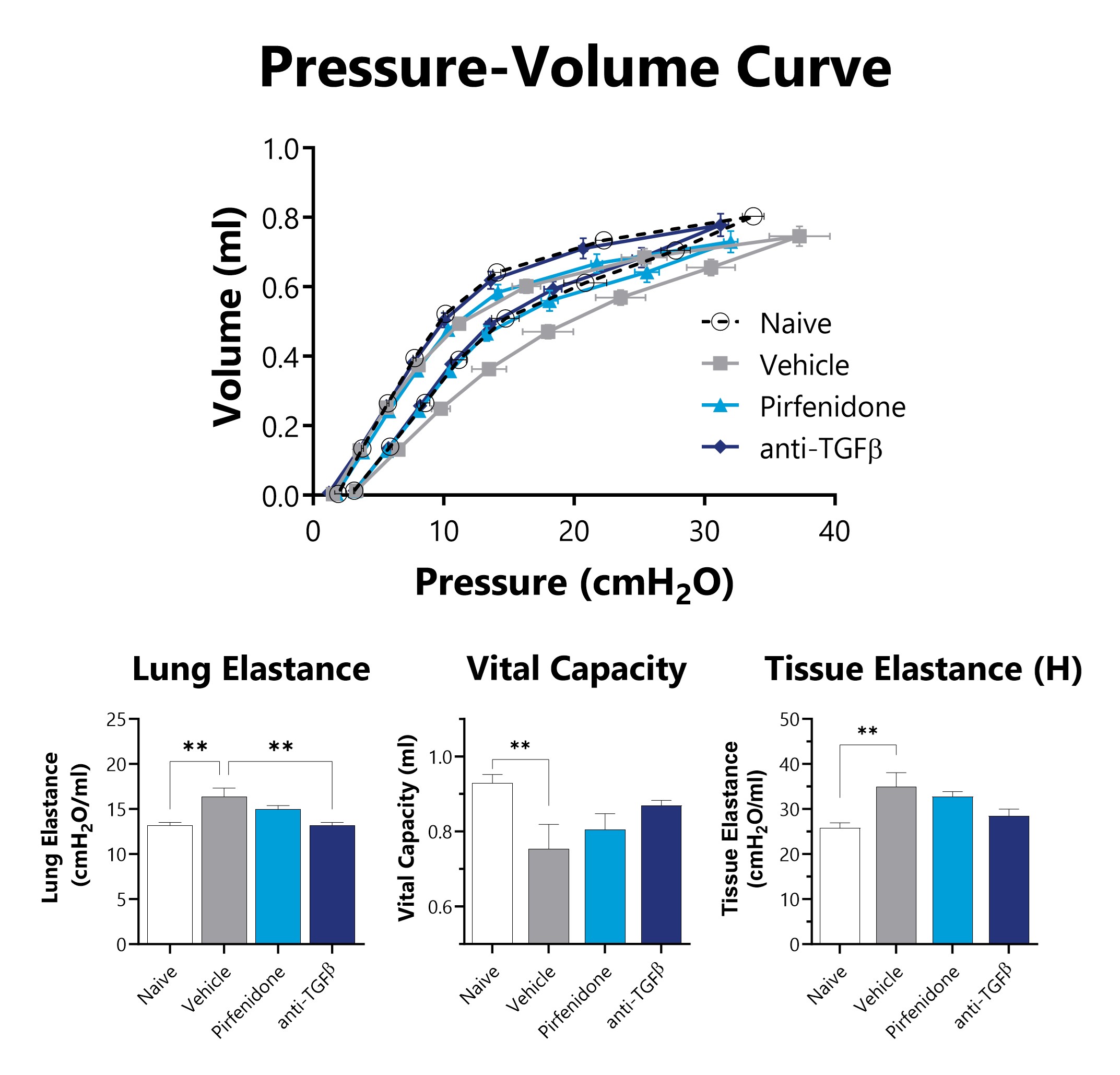
IT-Bleomycin administered animals have lung mechanics measured on Day 21 using a flexiVent mechanical ventilator. Improved lung function is observed in mice administered intratracheal bleomycin when treated with either pirfenidone or anti-TGFβ neutralizing antibody. (**p<0.01 compared to the naïve group)
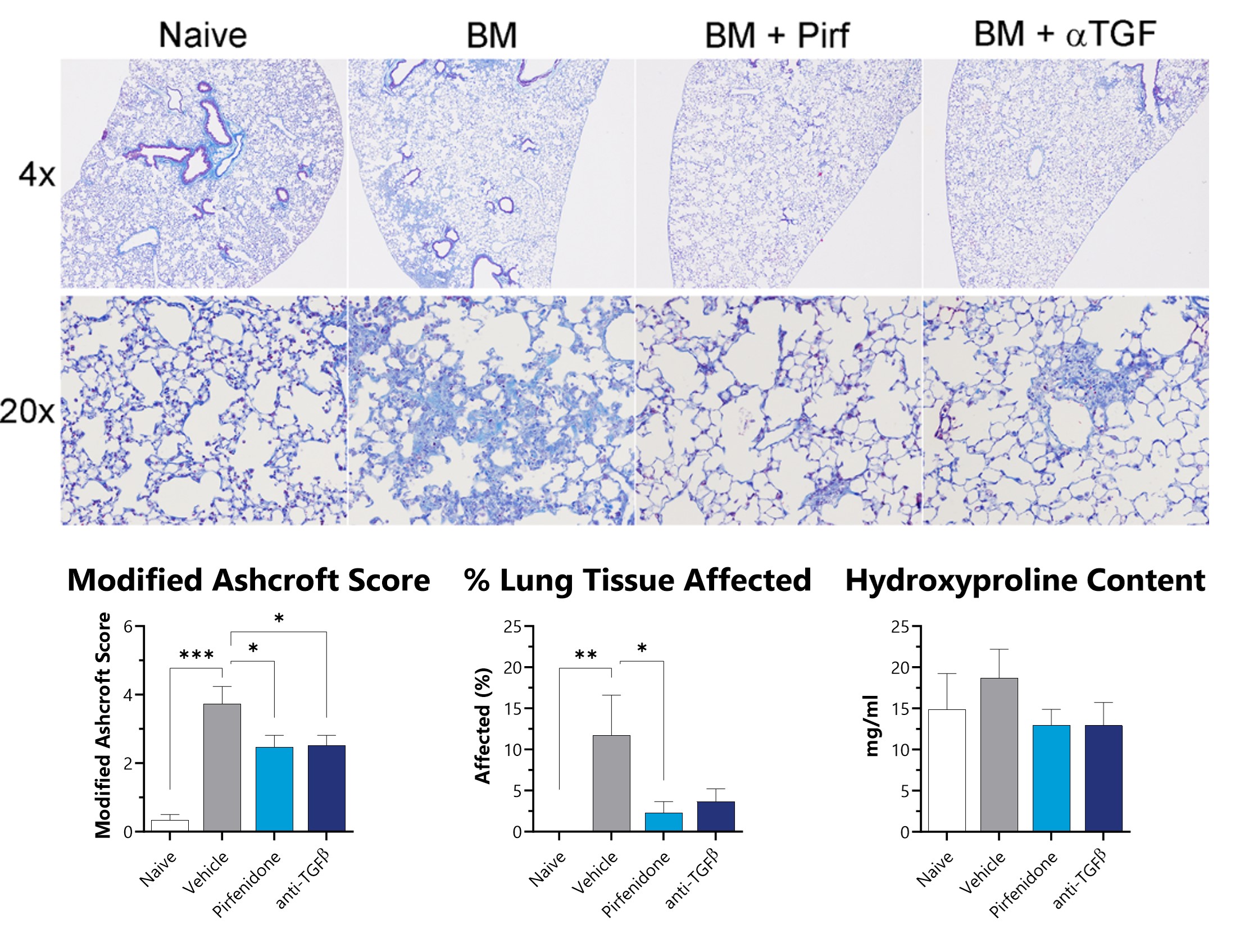
Lungs from IT-Bleomycin administered animals were collected on Day 21, processed for histopathology, and a modified Ashcroft score was assigned by a board-certified veterinary pathologist. Fixed tissue was also assessed for percent area of fibrotic lung tissue. Pulverized lung samples were assessed hydroxyproline. Lower levels of fibrosis are observed in mice administered intratracheal bleomycin when treated with either pirfenidone or anti-TGFβ neutralizing antibody. (*p<0.05; **p<0.01; ***p<0.001 compared to the naïve group)

Close

Pulmonary fibrosis is induced by subcutaneously (SC) administering bleomycin over the course of 28 days. To induce disease, an osmotic pump containing bleomycin is subcutaneously implanted in the backs of anesthetized mice. Animals are evaluated daily for body weight and respiratory status. Following planned euthanasia, lung weight, collagen content and fibrosis scores are determined.
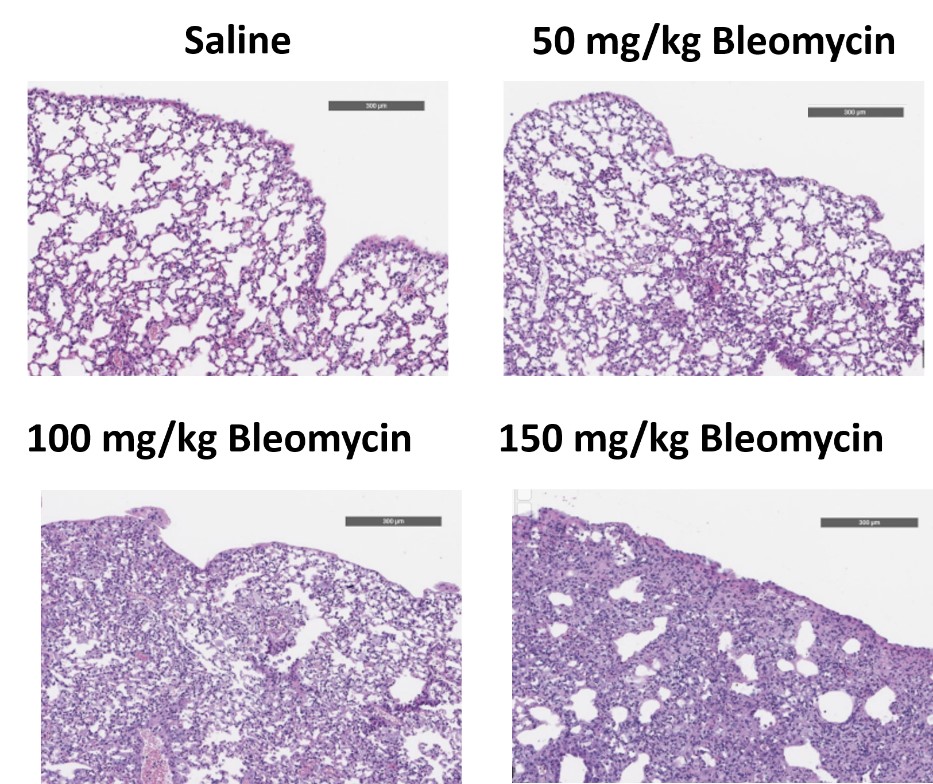
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and Day 28 lungs are processed for histopathology. H&E-stained lungs from animals administered saline or 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin.
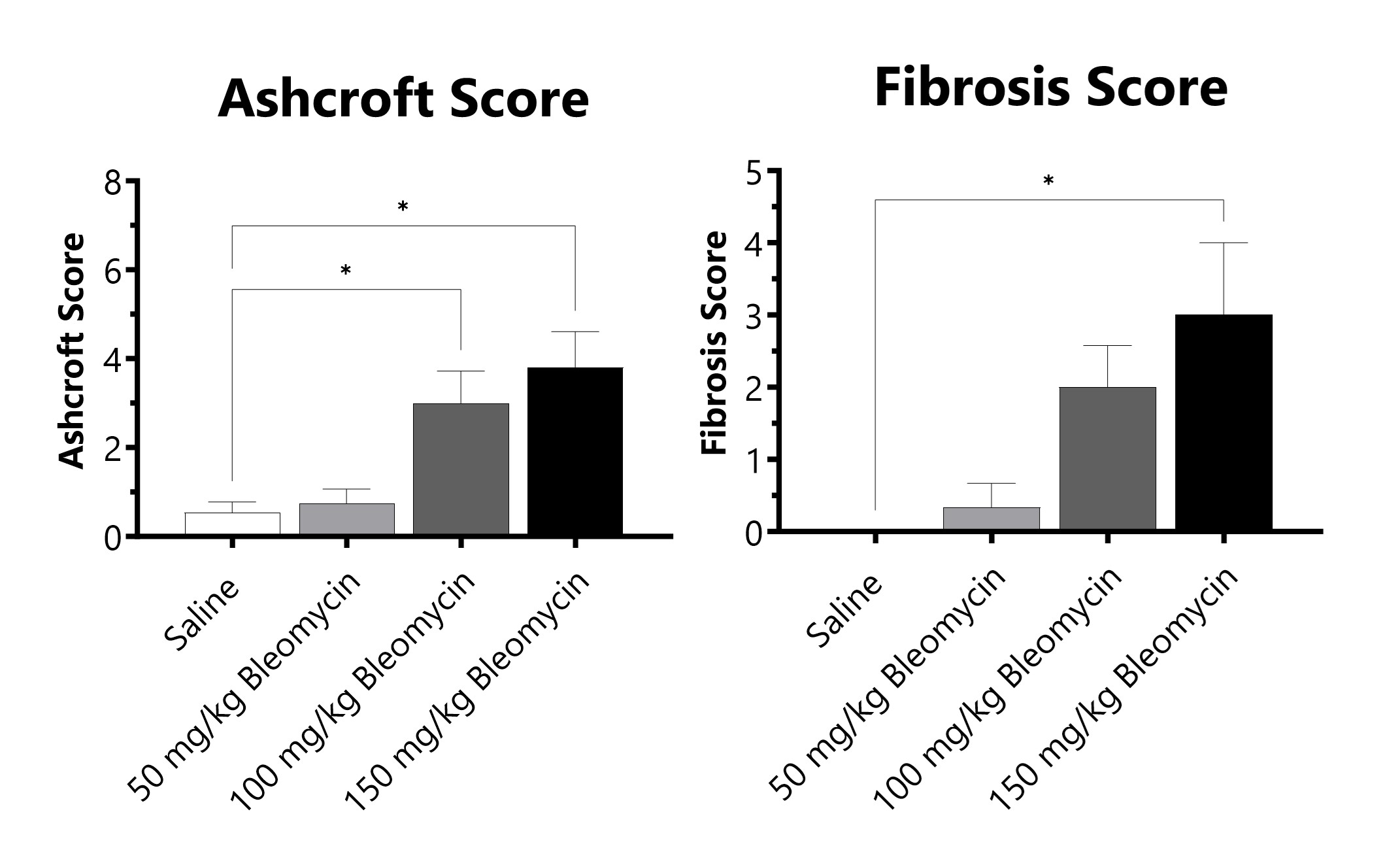
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump. Pathology assessment of Day 28 lungs from SC Osmotic Pump-Bleomycin administered animals. Ashcroft score is determined in H&E-stained tissue using a modified Ashcroft Scale graded from 0-8. Fibrosis score is determined in Masson’s Trichrome-stained tissue using a 0-5 semi-quantitative scoring scale of area staining positive for collagen. (*p<0.05 compared to the saline-control).
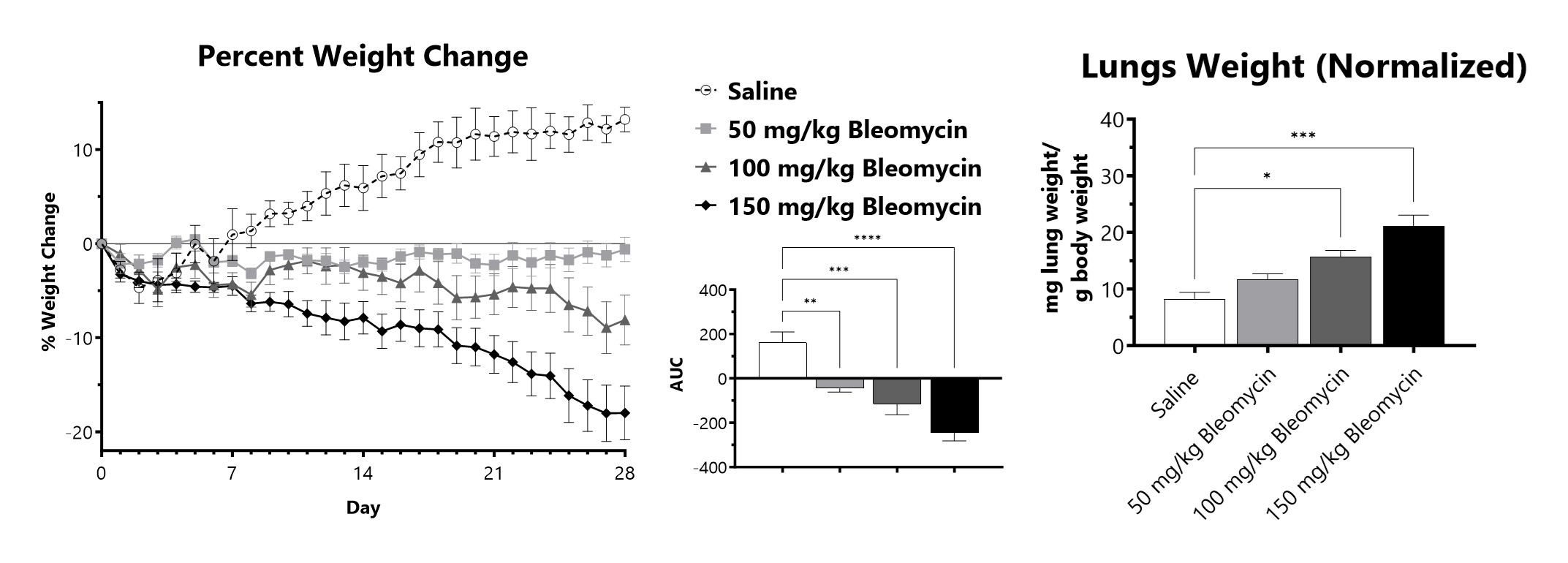
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump. Animals are weighed daily, and body weight compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. Wet lung weight normalized to Day 28 body weight of Day 28 lungs from SC Osmotic Pump-Bleomycin administered animals. (*p<0.05; ***p<0.001 compared to the saline-control).
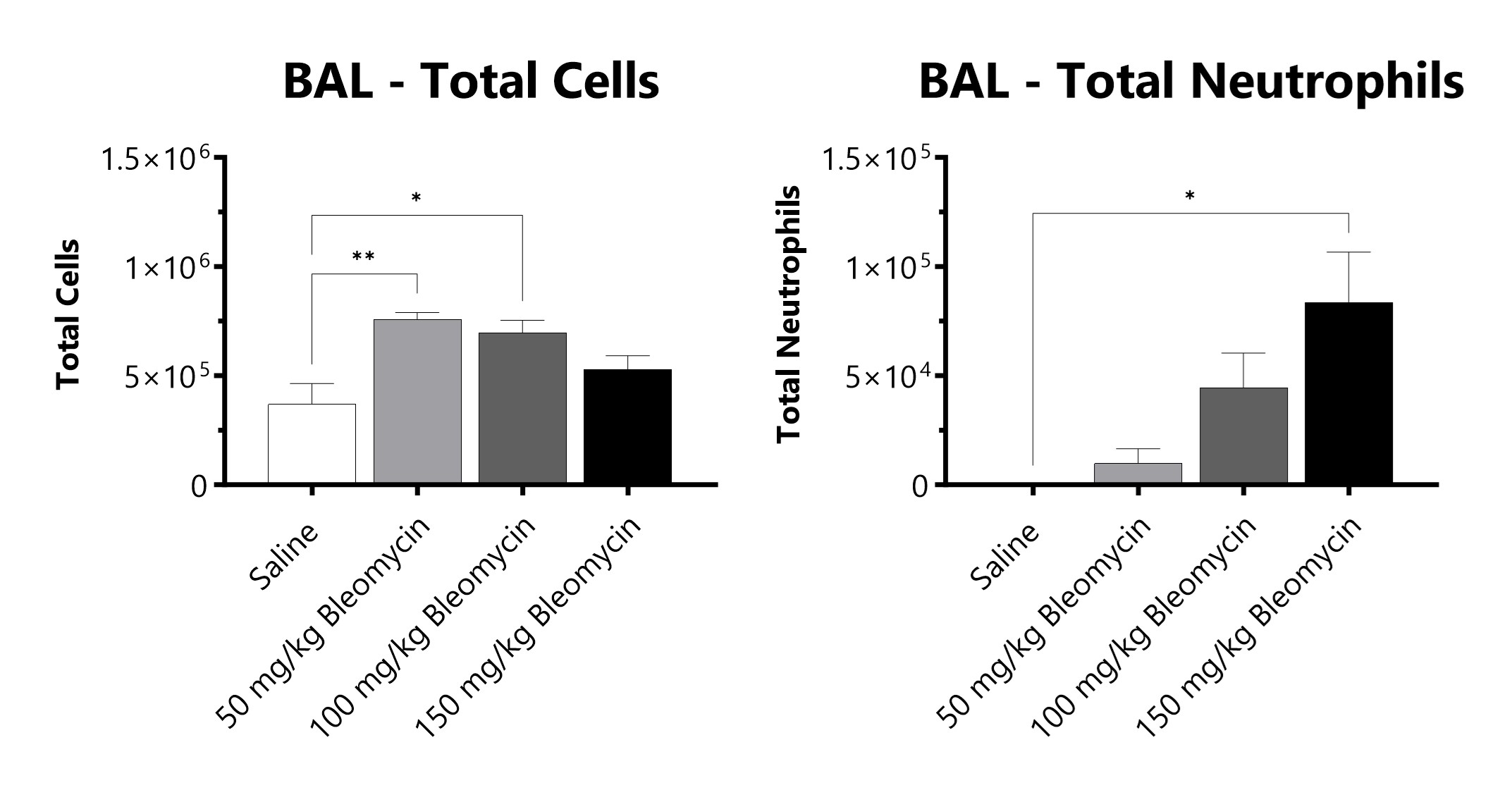
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and BAL fluid is assessed at 28 days for total cells and total neutrophils following administration of either 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin. (*p<0.05; **p<0.01 compared to the saline-control).
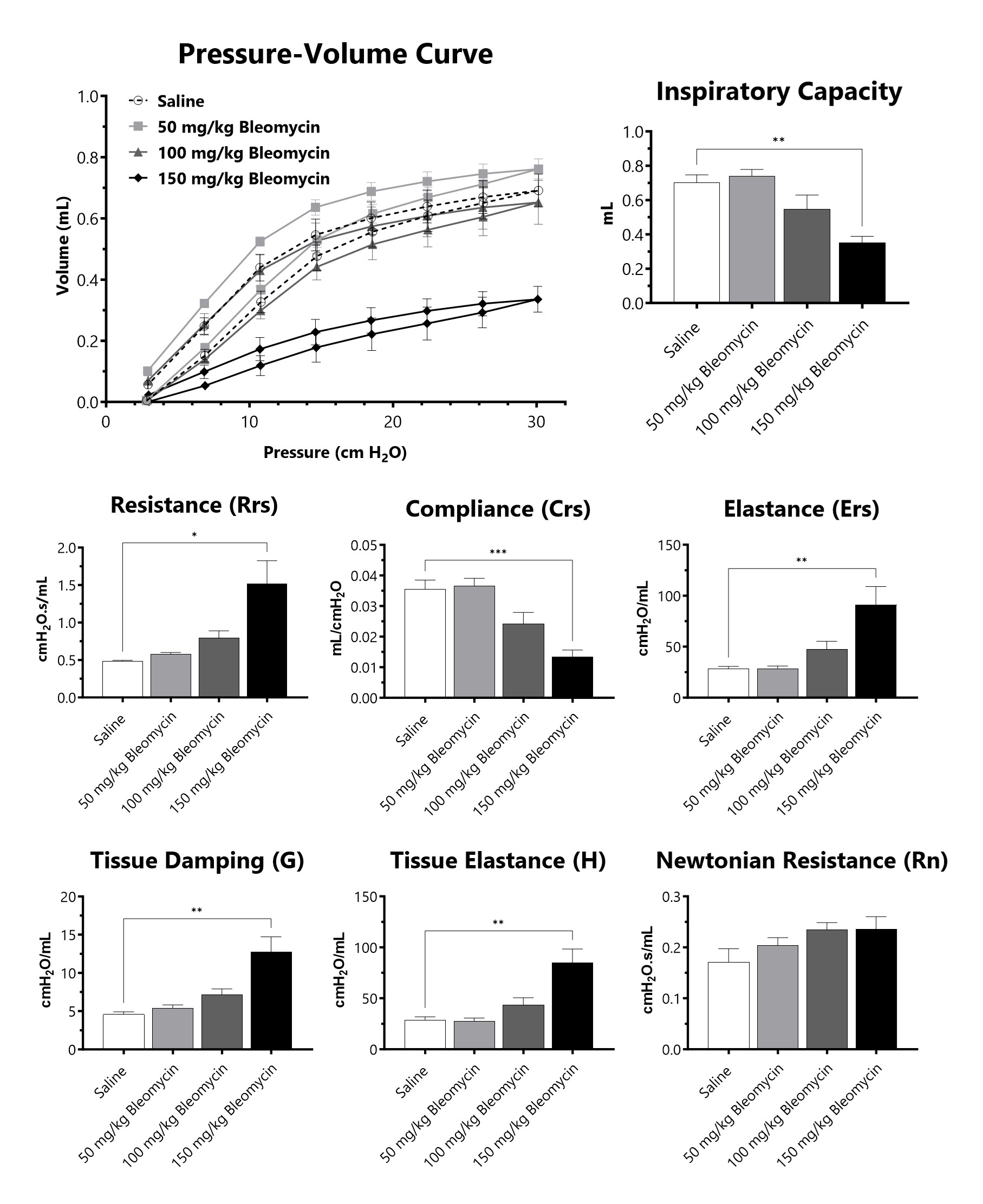
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and lung mechanics are assessed at 28 days following administration of either 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin. (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 compared to the saline-control).

Close

Pulmonary fibrosis can be induced by targeting a dose of radiation specifically to the thorax of a mouse, developing disease over the course of 4-6 months. While fibrosis is slow to develop in this model, changes in lung function can be detected as early as 8 weeks post-irradiation. After being anesthetized, a single dose of radiation is targeted to the thorax, while the remainder of the body is protected with a lead shield. Animals are evaluated daily for body weight and respiratory status. After animals are sacrificed, lung weight, collagen content and fibrosis scores are determined.
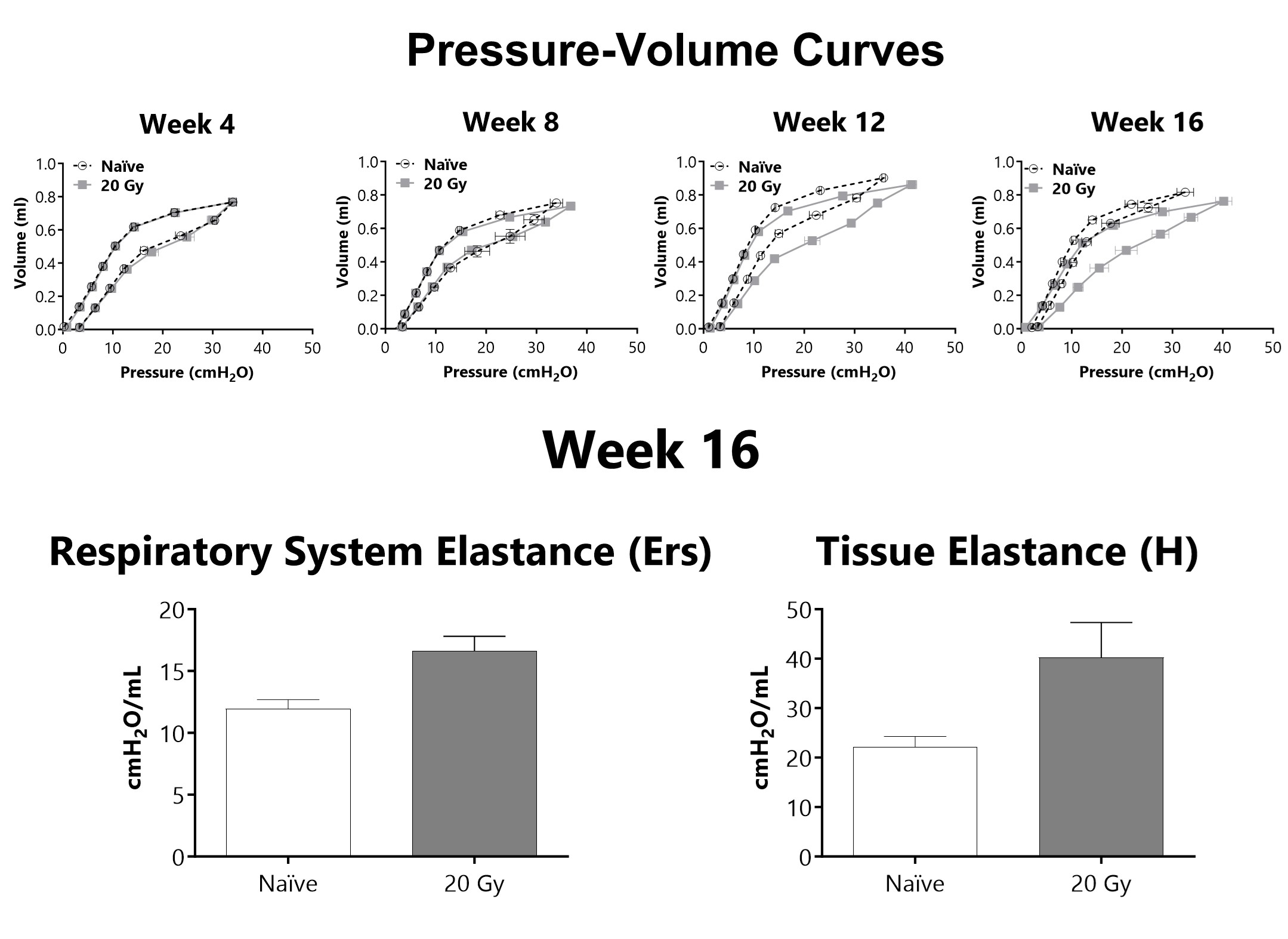
Lung mechanics are measured at either 4, 8, 12, or 16 weeks following administration of 20 Gy of radiation targeted to the thorax using a flexiVent mechanical ventilator. After 16 weeks, lung elastance (Ers) and tissue elastance (H) are increased in animals that received targeted radiation to the thorax.
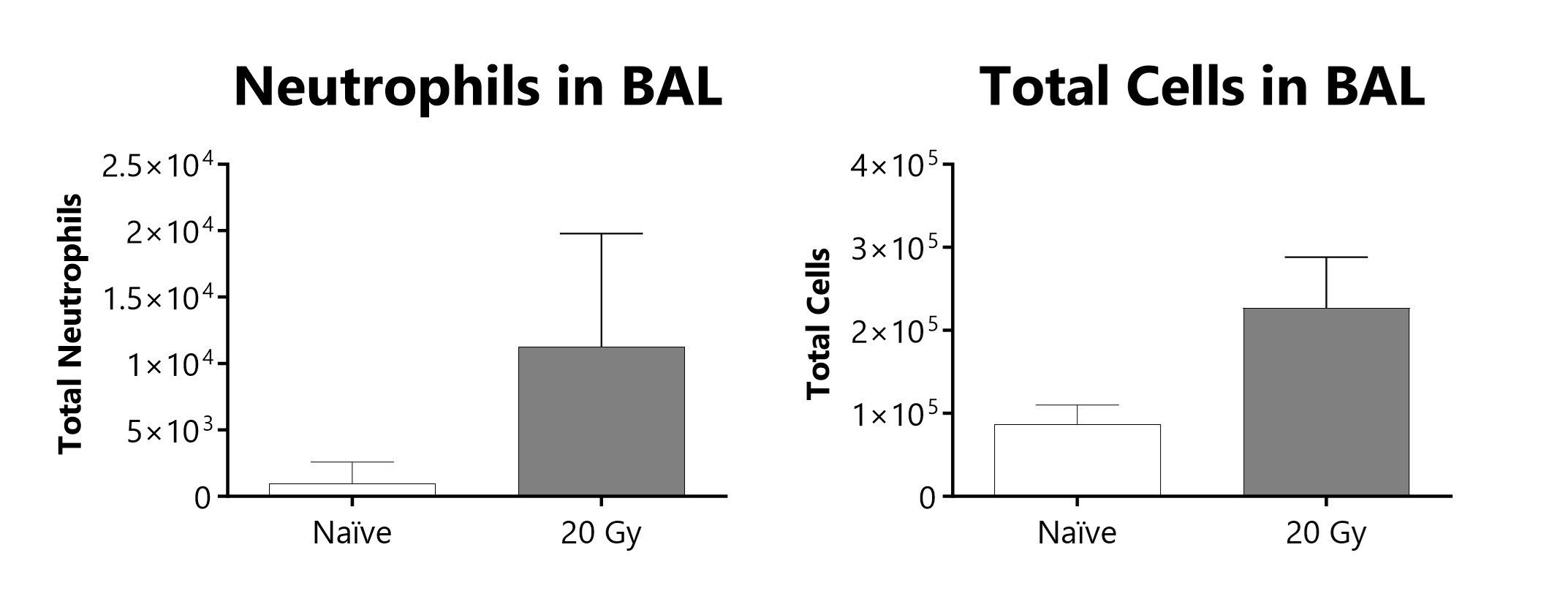
Total cells and neutrophils recovered in broncho-alveolar lavage fluid at week 16 of a radiation-induced pulmonary fibrosis model.
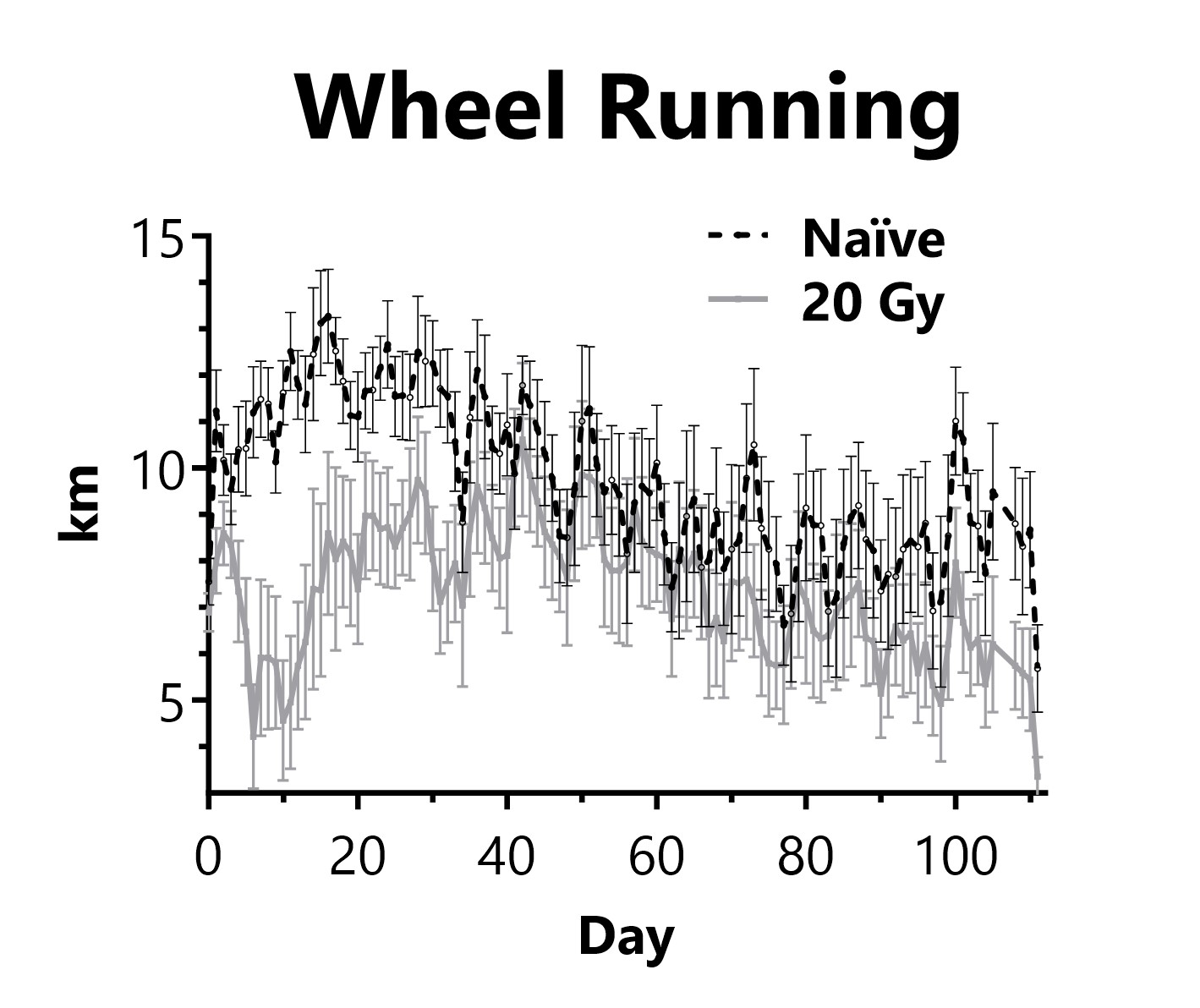
Animals are singly housed with a running wheel following administration of 20 Gy of radiation targeted to the thorax on Day 0. Daily distance traveled on the running wheel is recorded.

Close

At BioModels, we utilize a human in vitro model of TGF-β-induced fibrosis to screen potential therapeutics. TGFβ is added to confluent normal human lung fibroblasts (NHLF) for 48 hours. Supernatants and cell lysates are collected for endpoint analysis. Protein levels of Procollagen type 1-C peptide (PIP) and Plasminogen activator Inhibitor-1 (PAI-1) are determined to evaluate progression of fibrosis. Additionally, alpha smooth muscle actin (αSMA) is measured to indicate myofibroblast formation.
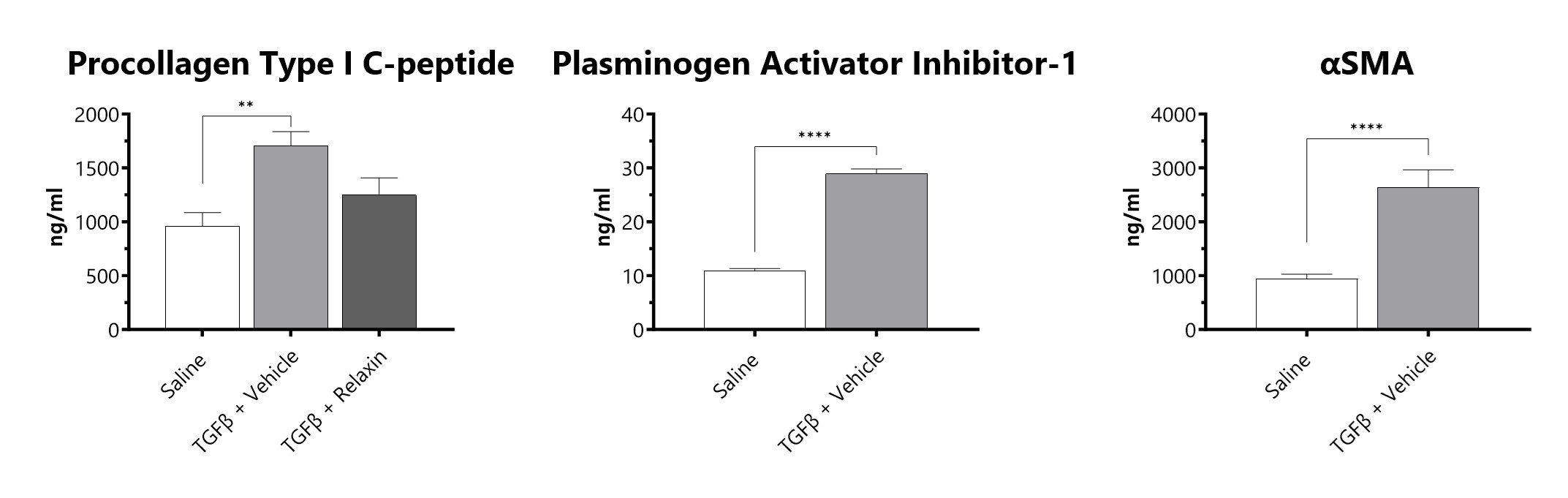
Confluent NHLFs are incubated for 48 hours with TGFβ. Levels of PIP and PAI-1 are assessed in cell culture supernatant and αSMA is measured in the cell lysate. (**p<0.01; ****p<0.0001 compared to the saline-control).

Close
Study Models

The murine model of bleomycin-induced dermal fibrosis is a highly utilized model of human dermal fibrosis. At BioModels, mice are administered daily subcutaneous (SC) injections of bleomycin for 28 days. Animals are evaluated daily for body weight changes. After animals are sacrificed, collagen thickness and content, fibrosis scores, and other markers of skin fibrosis are assessed.
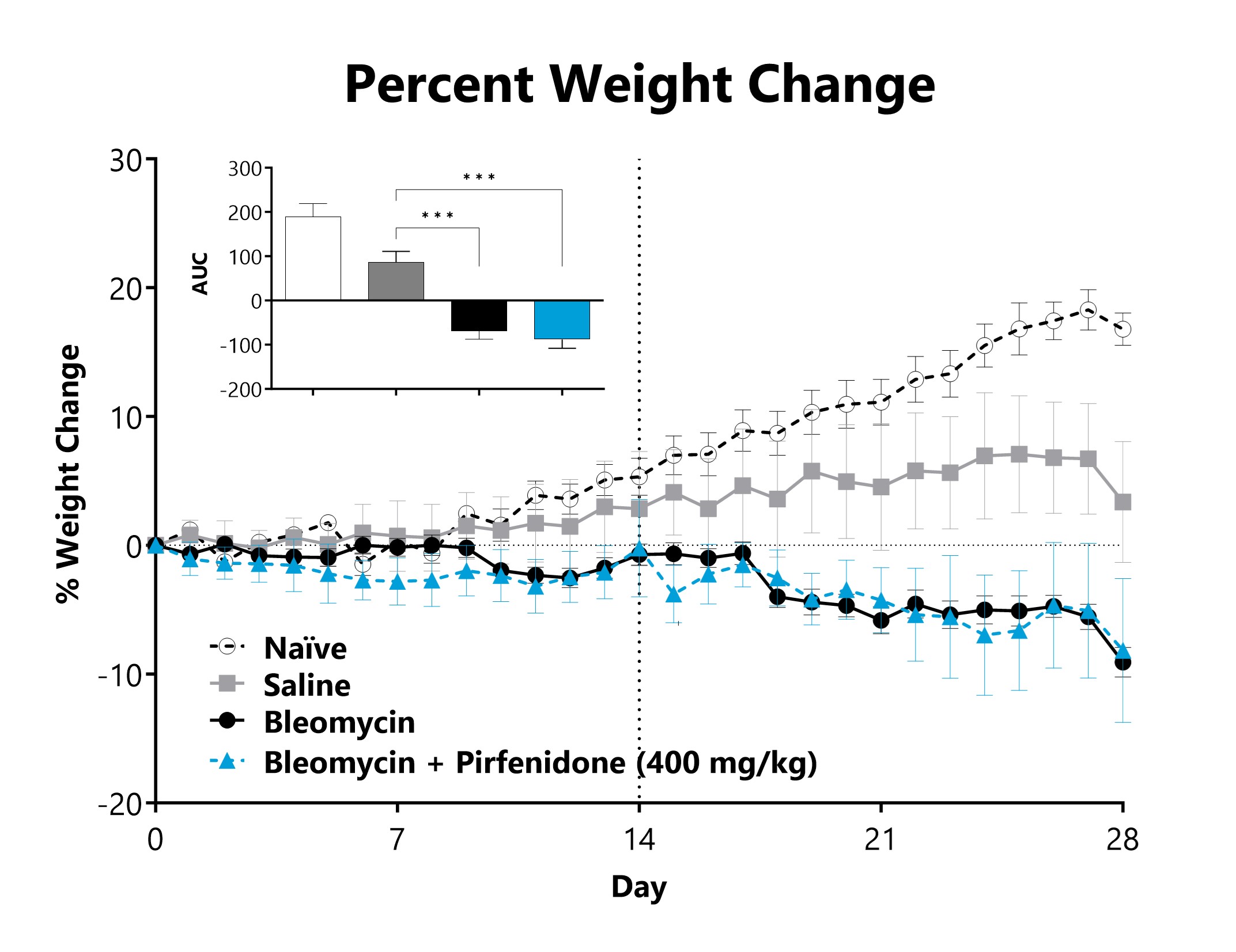
SC-Bleomycin administered animals are weighed daily, and body weight compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. (***p<0.001 compared to the saline-control).
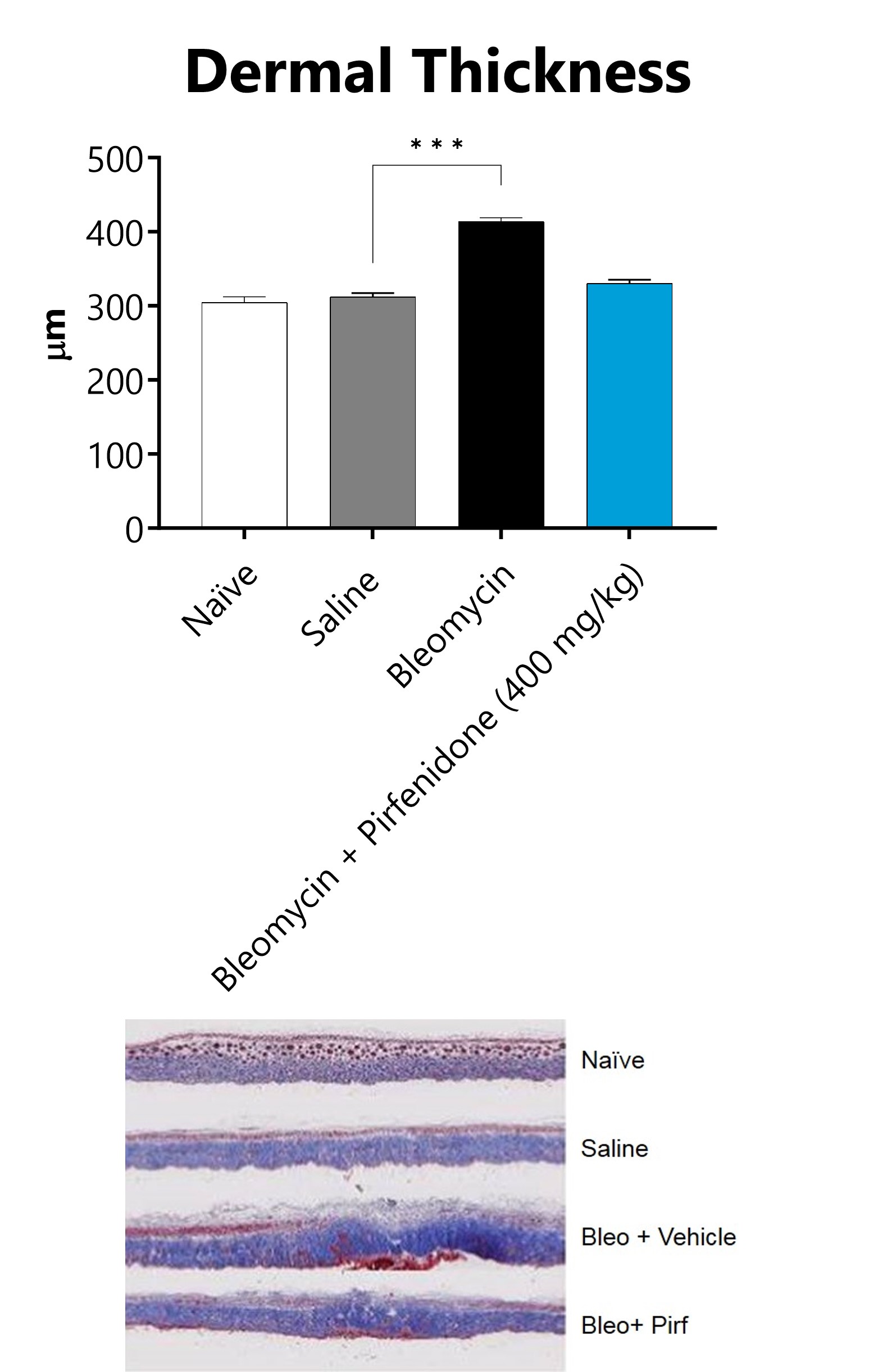
Skin from SC-Bleomycin administered animals is collected on Day 28, processed for histopathology, and dermal thickness measured. Representative images of Masson’s Trichrome staining. (***p<0.001 compared to the saline control group).

Close

In the sclerodermatous chronic GvHD model at BioModels, dermal fibrosis appears beginning at day 30. Animals are evaluated daily for body weight and GVHD scores. After sacrifice, collagen thickness and content, fibrosis scores, and other markers of skin fibrosis are determined.

Close

In the fractionated radiation-induced dermal fibrosis model, the back of the mouse is shaved and/or depilated and the skin is temporarily tented such that it can be selectively targeted with radiation while the rest of the mouse is protected by a lead shield. Animals receive 6 fractions of radiation on a schedule of 3 days on, 2 days off. The progression of disease, which can also be observed in an acute radiation model, displays with mild erythema typically observed 8-10 days following radiation exposure, with dermatitis severity peaking between Days 12-16 (depending on the dose of radiation), and with desquamation of approximately 50% of the irradiated area. Peak levels of fibrosis are observed at 36 days following the start of radiation. Endpoints include dermatitis severity scores, collagen content measurement, and histological analyses.
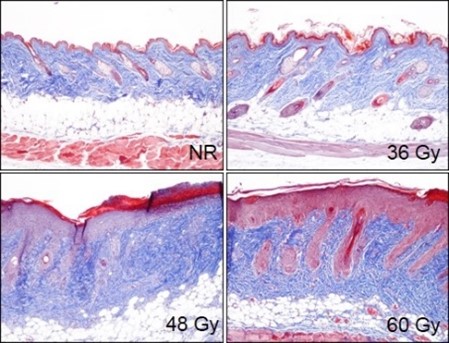
Animals receive a targeted fractionated radiation dose of either 36 Gy, 48 Gy, or 60 Gy to tented back skin, starting on Day 0. On Day 36 skin tissue is collected and processed for histopathology assessment. Representative Masson’s trichrome-stained samples from non-radiated (NR) animals and animals receiving a total dose of radiation of either 36 Gy, 48 Gy, or 60 Gy.

Close

Dermal fibrosis is also induced by subcutaneously (SC) administering bleomycin over the course of 28 days. To induce disease, an osmotic pump containing bleomycin is subcutaneously implanted in the backs of anesthetized mice. Animals are evaluated daily for body weight change. After animals are sacrificed, collagen content and fibrosis scores are determined in skin.
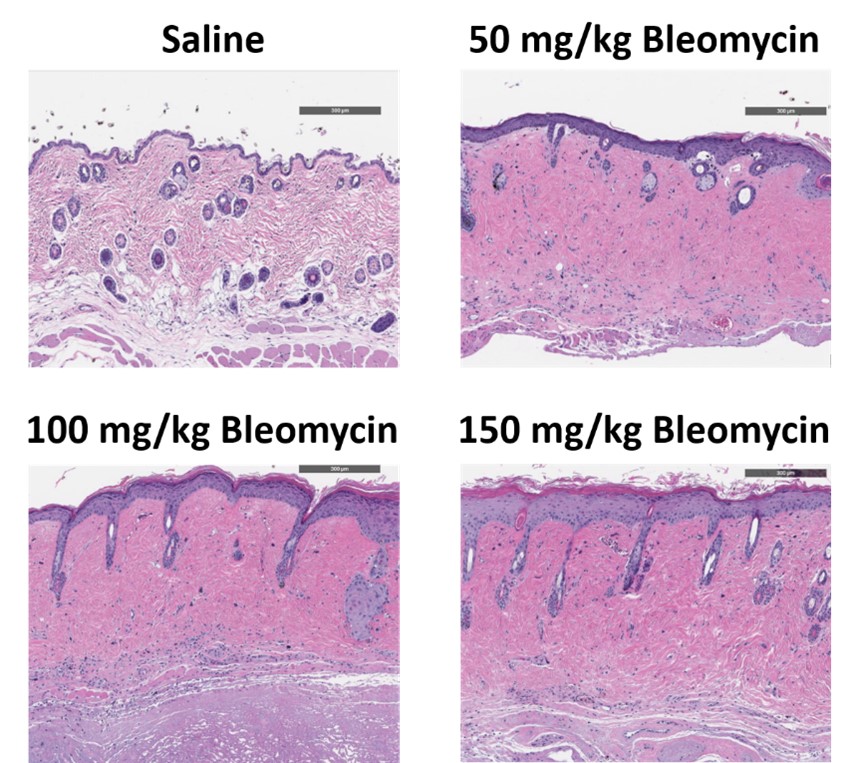
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and Day 28 skin samples are processed for histopathology. H&E-stained skin from animals administered saline or 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin.
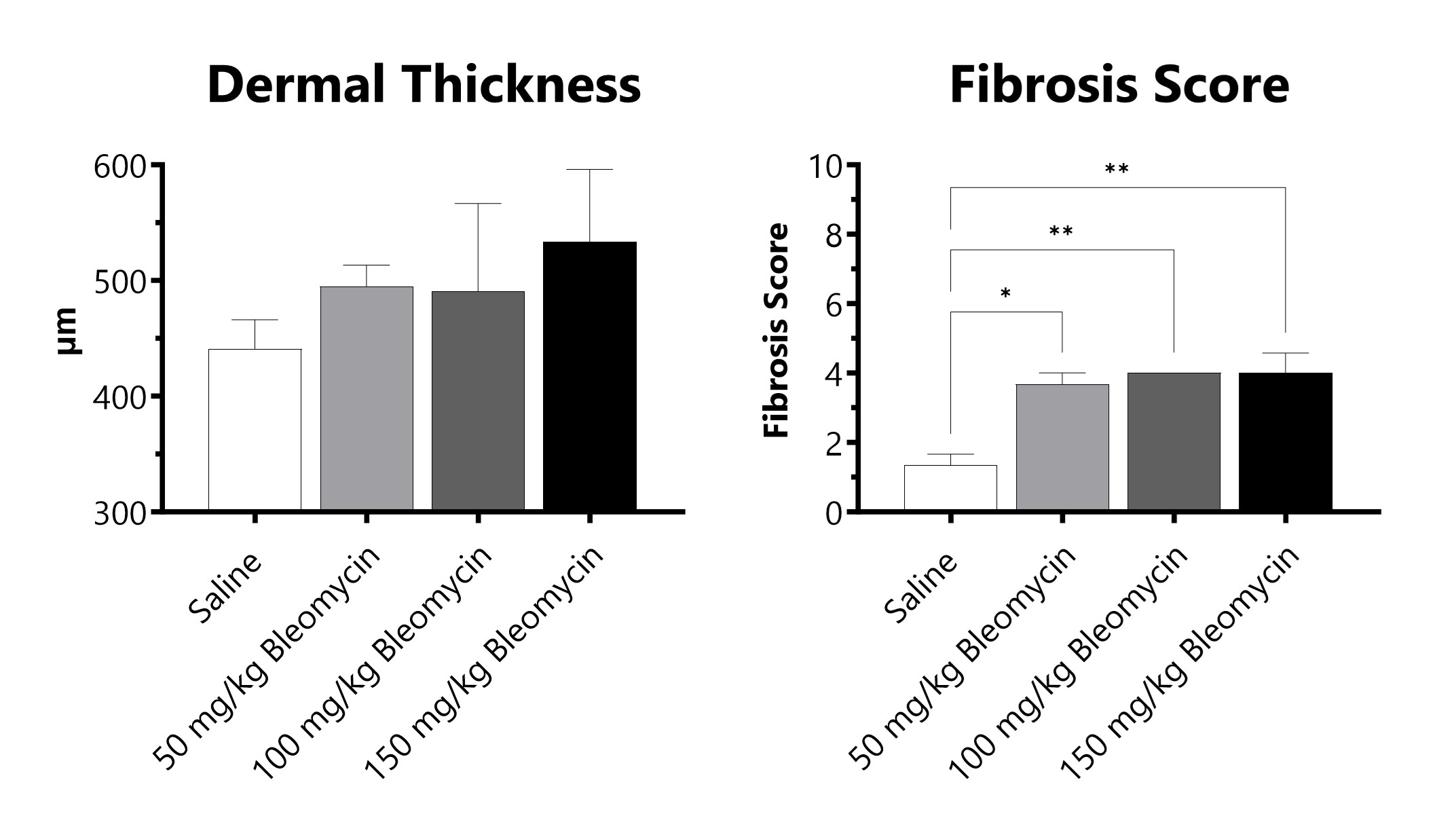
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump. Pathology assessment of Day 28 skin from SC Osmotic Pump-Bleomycin administered animals is shown. Dermal thickness is measured, and fibrosis score is determined in Masson’s Trichrome-stained tissue using a 0-5 semi-quantitative scoring scale of area staining positive for collagen. (*p<0.05; **p<0.01 compared to the saline-control).

Close
Study Models

Pulmonary fibrosis can be induced by targeting a dose of radiation specifically to the thorax of a mouse, developing disease over the course of 4-6 months. While fibrosis is slow to develop in this model, changes in lung function can be detected as early as 8 weeks post-irradiation. After being anesthetized, a single dose of radiation is targeted to the thorax, while the remainder of the body is protected with a lead shield. Animals are evaluated daily for body weight and respiratory status. After animals are sacrificed, lung weight, collagen content and fibrosis scores are determined.
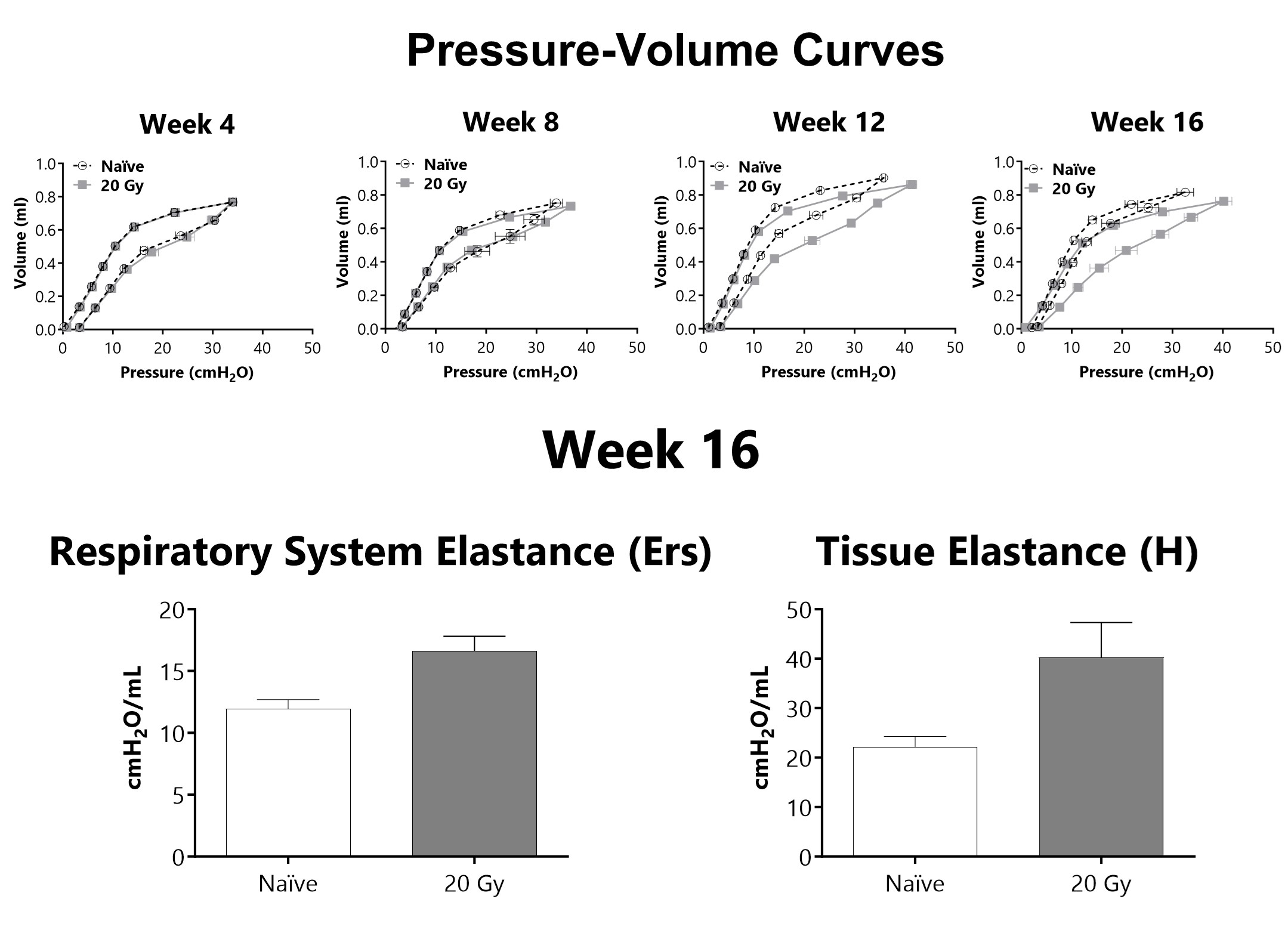
Lung mechanics are measured at either 4-, 8-, 12-, or 16-weeks following administration of 20 Gy of radiation targeted to the thorax using a flexiVent mechanical ventilator. After 16 weeks, lung elastance (Ers) and tissue elastance (H) are increased in animals that received targeted radiation to the thorax.
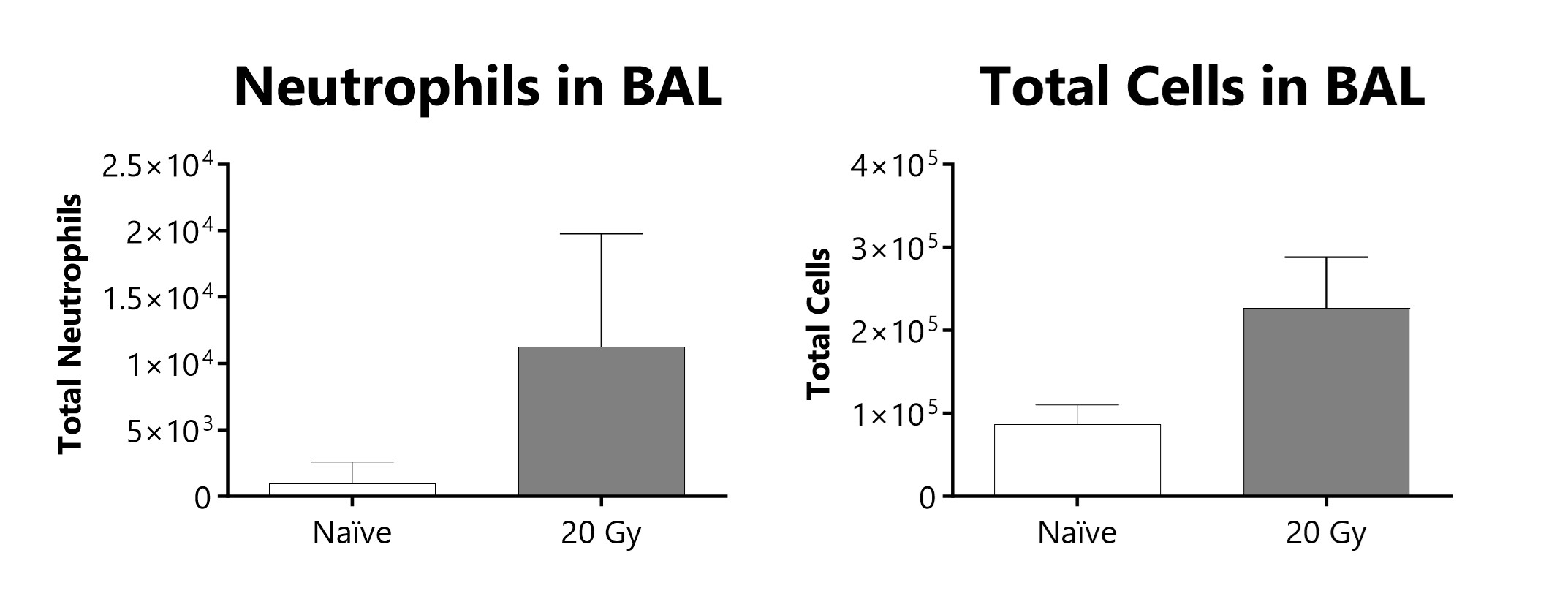
Total cells and neutrophils recovered in broncho-alveolar lavage fluid at week 16 of a radiation-induced pulmonary fibrosis model.
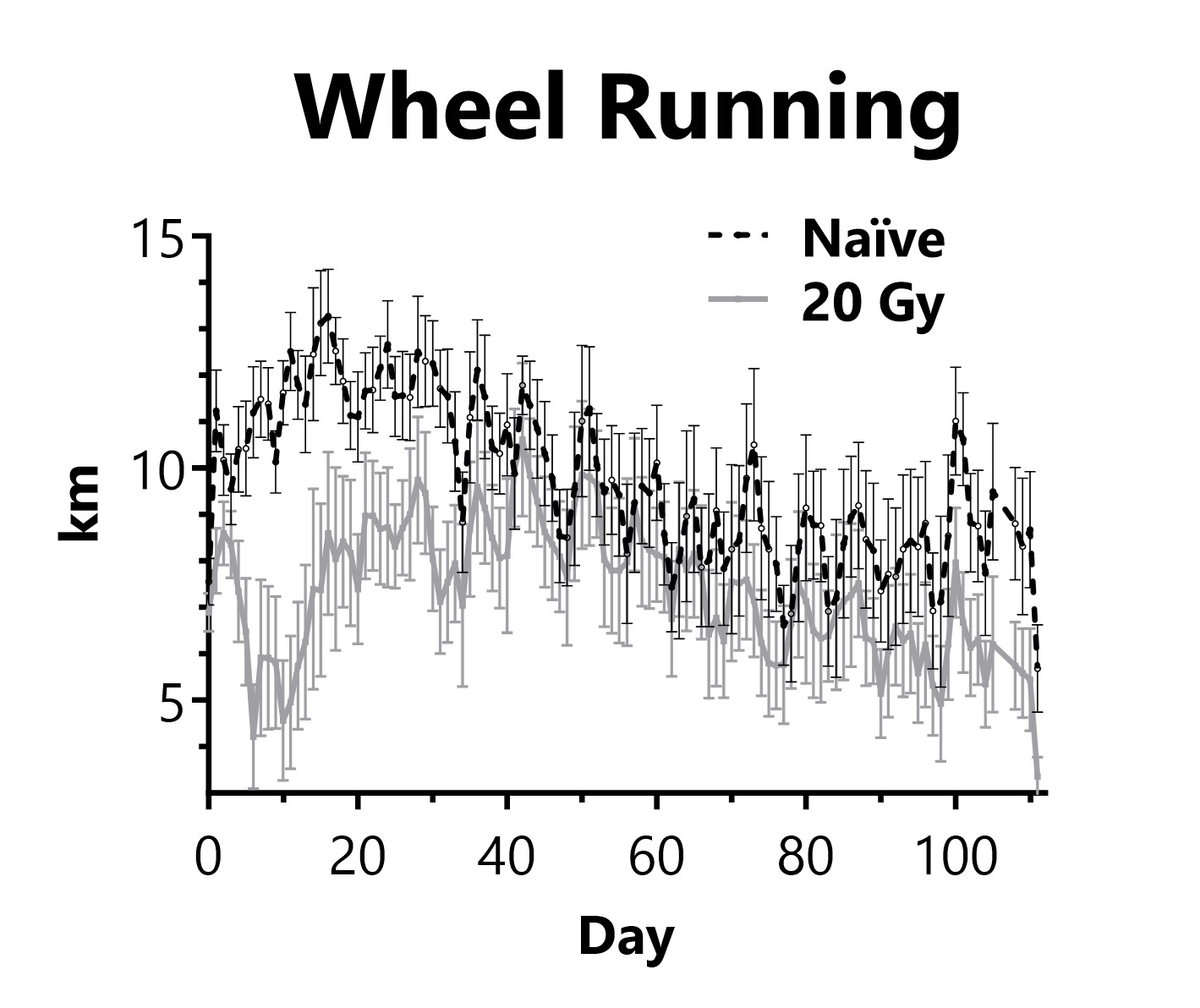
Animals are singly housed with a running wheel following administration of 20 Gy of radiation targeted to the thorax on Day 0. Daily distance traveled on the running wheel is recorded.

Close

Hamsters provide an excellent model for radiation-induced fibrosis because of several biological similarities to humans. One distinct advantage of the model is that the cheek pouch is pliable and can be extracted, allowing for radiation to be targeted to the cheek pouch while the rest of the animal is shielded. The result is formation of fibrotic tissue on the cheek pouch approximately 16 days following radiation. Primary end points in this model include collagen content measurement and histology.
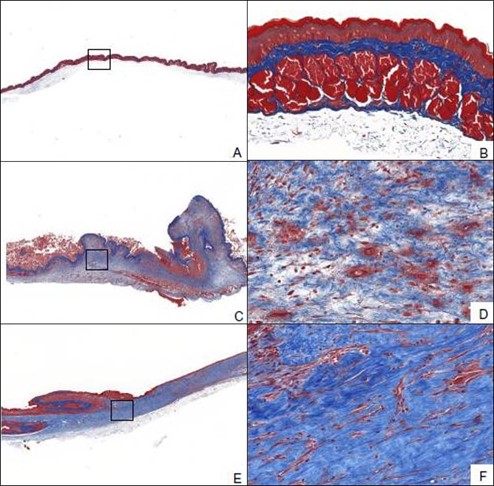
Animals receive a targeted radiation dose of 40 Gy to the cheek pouch on Day 0. At Day 0, 16, and 28 cheek tissue is collected and processed for histopathology assessment. Representative Masson’s trichrome-stained samples from Day 0 (A, B), Day 16 (C, D), and Day 28 (E, F) after radiation.

Close

In the fractionated radiation-induced dermal fibrosis model, the back of the mouse is shaved and/or depilated and the skin is temporarily tented such that it can be selectively targeted with radiation while the rest of the mouse is protected by a lead shield. Animals receive 6 fractions of radiation on a schedule of 3 days on, 2 days off. The progression of disease, which can also be observed in an acute radiation model, displays with mild erythema typically observed 8-10 days following radiation exposure, with dermatitis severity peaking between Days 12-16 (depending on the dose of radiation), and with desquamation of approximately 50% of the irradiated area. Peak levels of fibrosis are observed at 36 days following the start of radiation. Endpoints include dermatitis severity scores, collagen content measurement, and histological analyses.
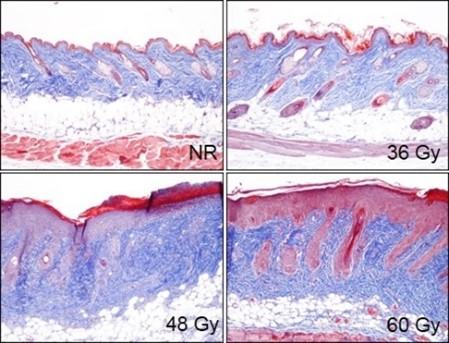
Animals receive a targeted fractionated radiation dose of either 36 Gy, 48 Gy, or 60 Gy to tented back skin, starting on Day 0. At Day 36 skin tissue is collected and processed for histopathology assessment. Representative Masson’s trichrome-stained samples from non-radiated (NR) animals and animals receiving a total dose of radiation of either 36 Gy, 48 Gy, or 60 Gy.

Close
Study Models


Liver samples from CCl4 administered animals are processed for histopathology and scored for levels of fibrosis on a scale of 0-5 (0 = normal liver, 1=minimal increase in fibrosis, 2=moderate increase in fibrosis, 4=marked increase in fibrosis, 5=severe increase in fibrosis/cirrhotic. (****p<0.0001 compared to the corn oil control group). Histopathology performed by Dallas Tissue Research.
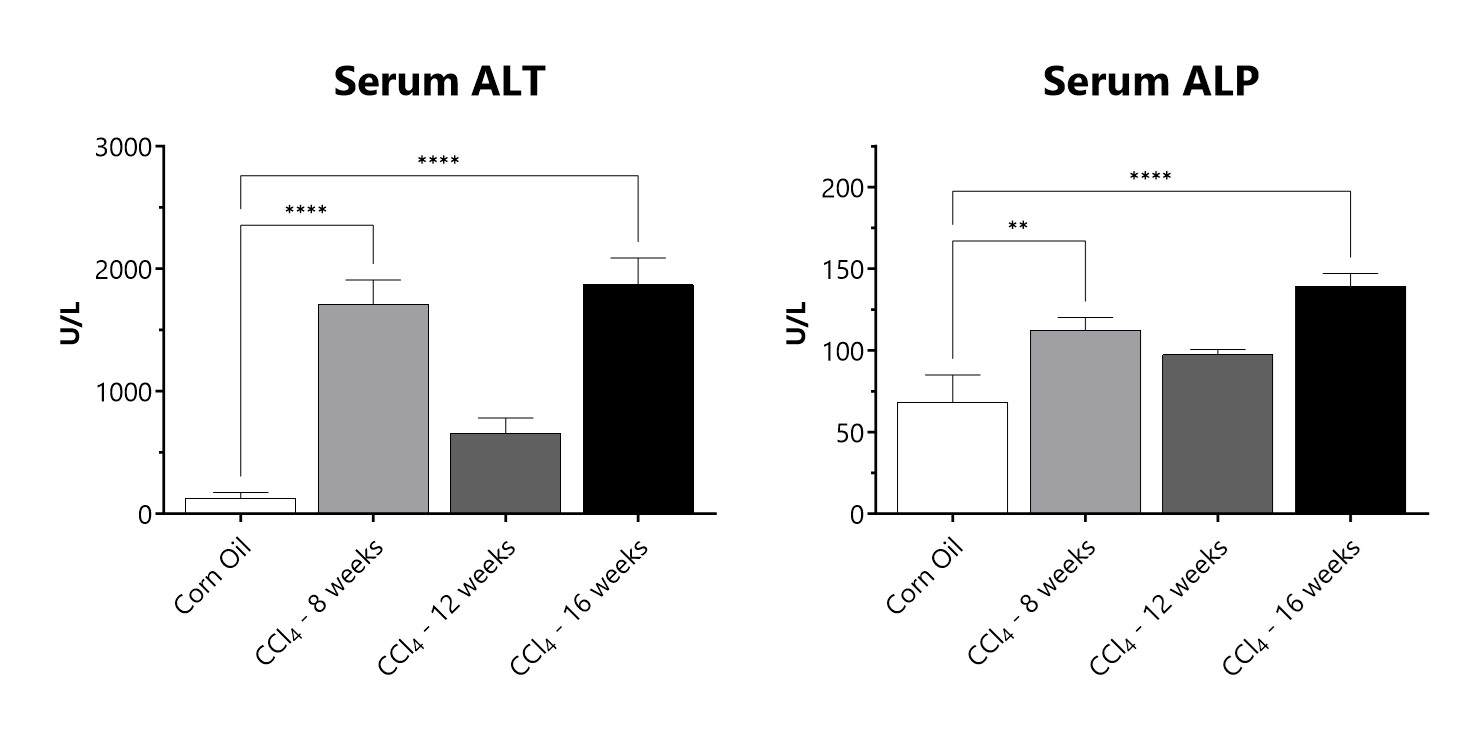
Serum samples from CCl4 administered animals are assessed for levels of alanine transaminase (ALT) and alkaline phosphatase (ALP). (**p<0.01; ****p<0.0001 compared to the corn oil control group)
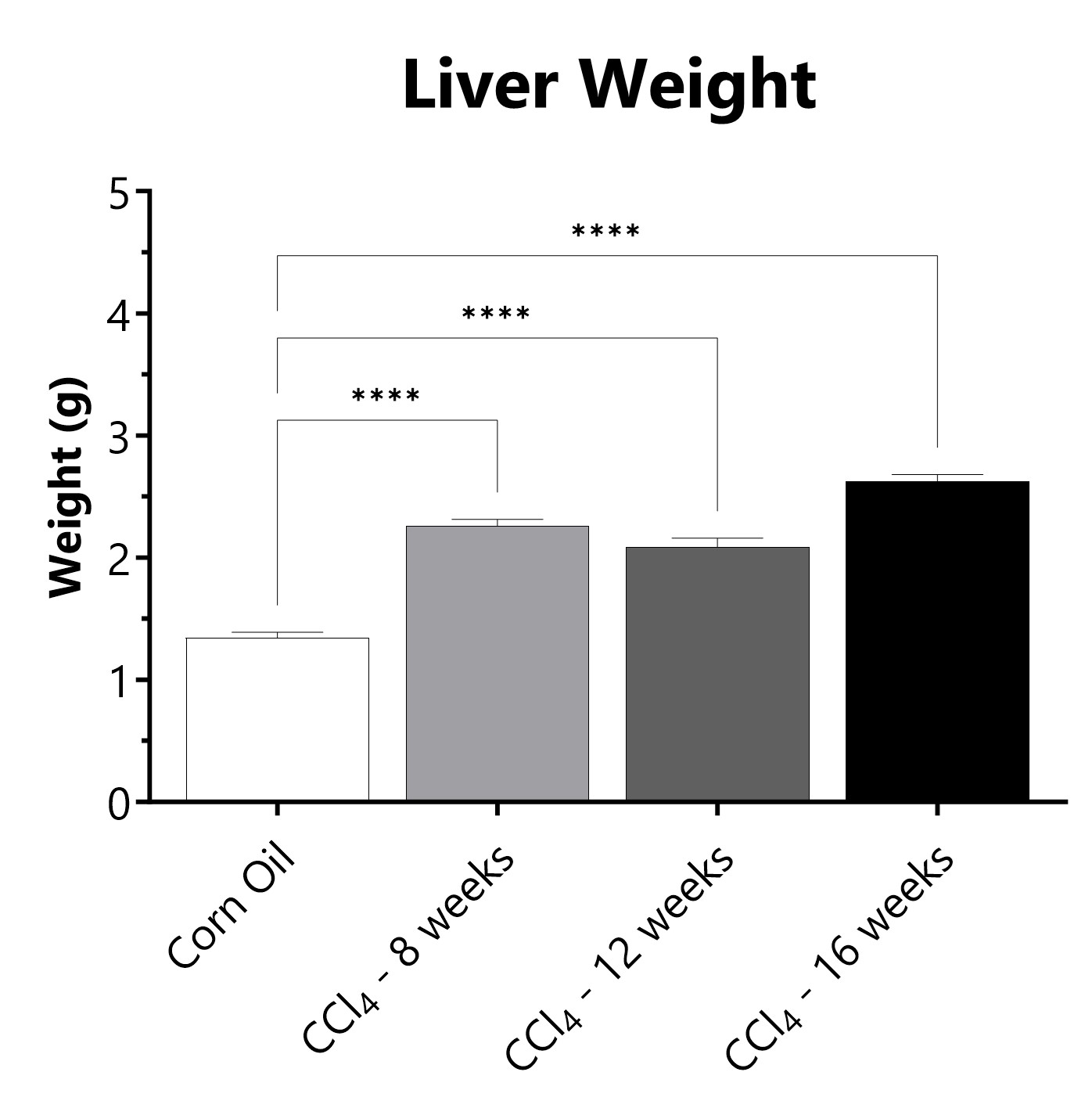
Following euthanasia, the liver is excited and weighed. (****p<0.0001 compared to the corn oil control group)

Close

Feeding mice an Amylin liver NASH (AMLN) diet leads to obesity, fatty liver, and after longer amounts of time both liver inflammation and fibrosis and has become a widely used model for nonalcoholic steatohepatitis (NASH). NASH is a severe form of nonalcoholic fatty liver disease (NAFLD), a condition in which the liver builds up excessive fat deposits which can progress to fibrosis, cirrhosis, and hepatocellular carcinoma.

Close
Study Models

Experimental Unilateral Ureter Obstruction (UUO) represents a model for obstructive nephropathy and allows insight into the development of interstitial fibrosis which is a common characteristic of many chronic nephropathies. Important markers of renal fibrosis, such as interstitial fibroblasts, interstitial volume, mRNA and protein expression for collagen I, are all increased in UUO animals, making the UUO model a good experimental system for studying fibrosis. At BioModels, disease is induced when UUO is performed by ligating the ureter below the left kidney of a mouse on Day 0. In order to assess the development of disease, the kidneys are removed (for histological assessment of fibrosis) and blood is collected between day 7 and 14.
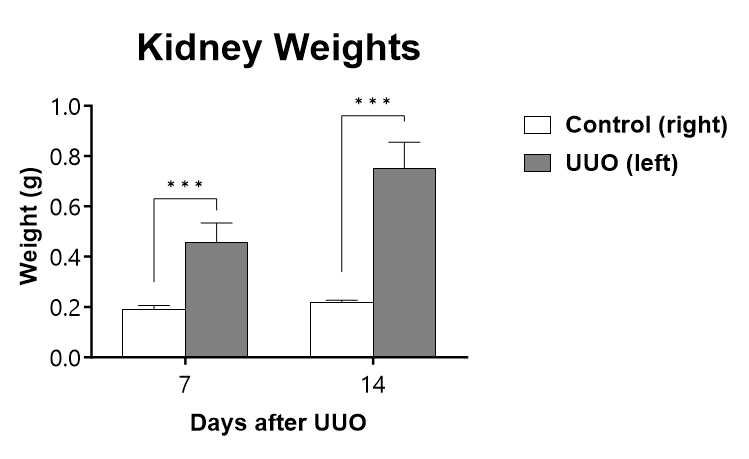
The UUO procedure is performed on C57Bl/6 mice on Day 0 and kidneys are collected and weighed at the specified endpoint. (***p<0.001 compared to the contralateral-control kidney).
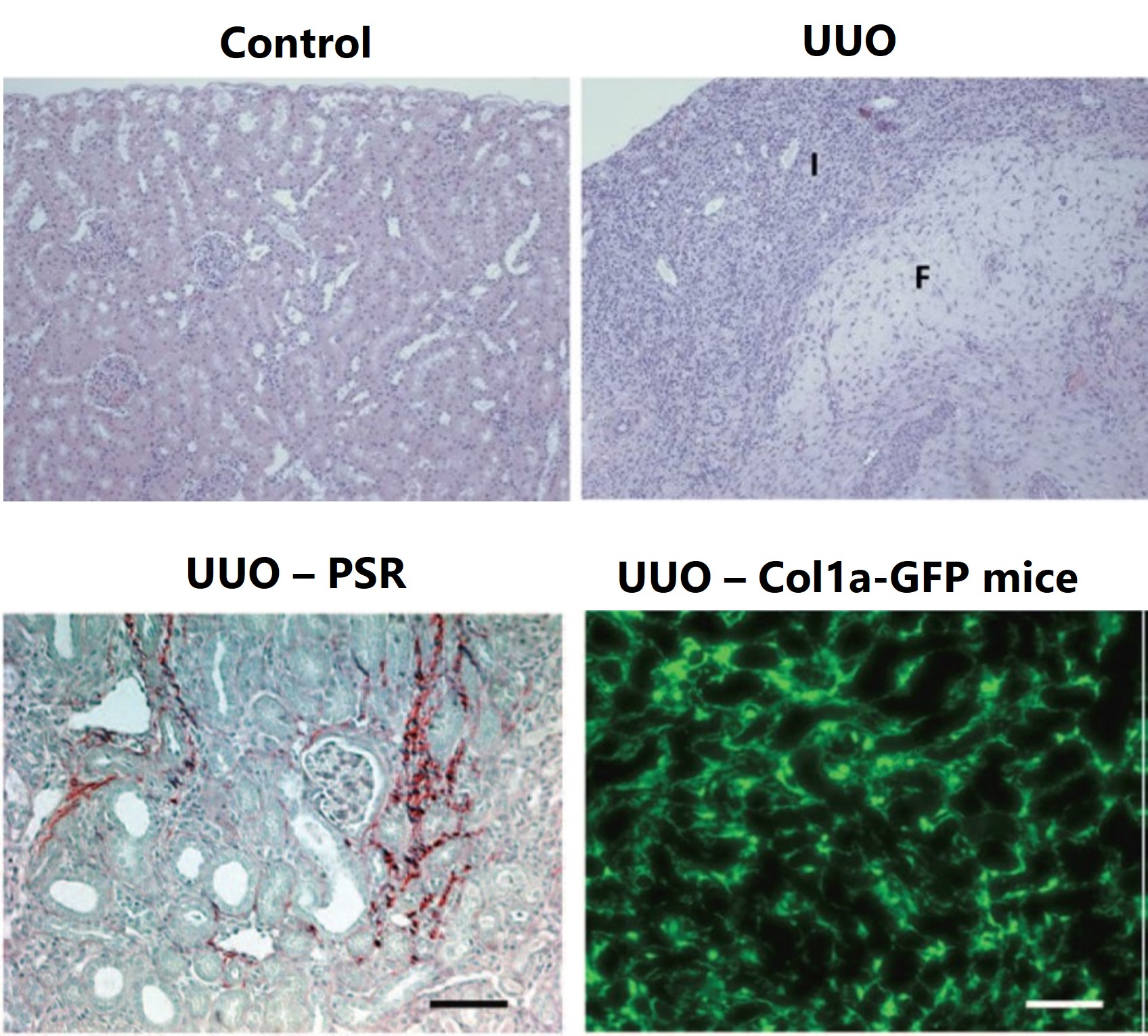
The UUO procedure is performed on C57Bl/6 mice on Day 0 and kidneys are collected for histopathology assessment in H&E-stained sections. Day 10 representative non-lesioned kidney cortex in control kidney and areas of inflammation (I) and fibrosis (F) in UUO kidney are displayed. Day 14 representative images of UUO kidney are shown with picrosirius red staining (bottom left). Day 14 representative images of UUO kidney from Col1a-GFP mice (bottom right).

Close

Dermal and pulmonary fibrosis are induced by subcutaneously (SC) administering bleomycin over the course of 28 days. To induce disease, an osmotic pump containing bleomycin is subcutaneously implanted in the backs of anesthetized mice. Animals are evaluated daily for body weight and respiratory status. After animals are sacrificed, lung weight, collagen content and fibrosis scores are determined in both lungs and skin.
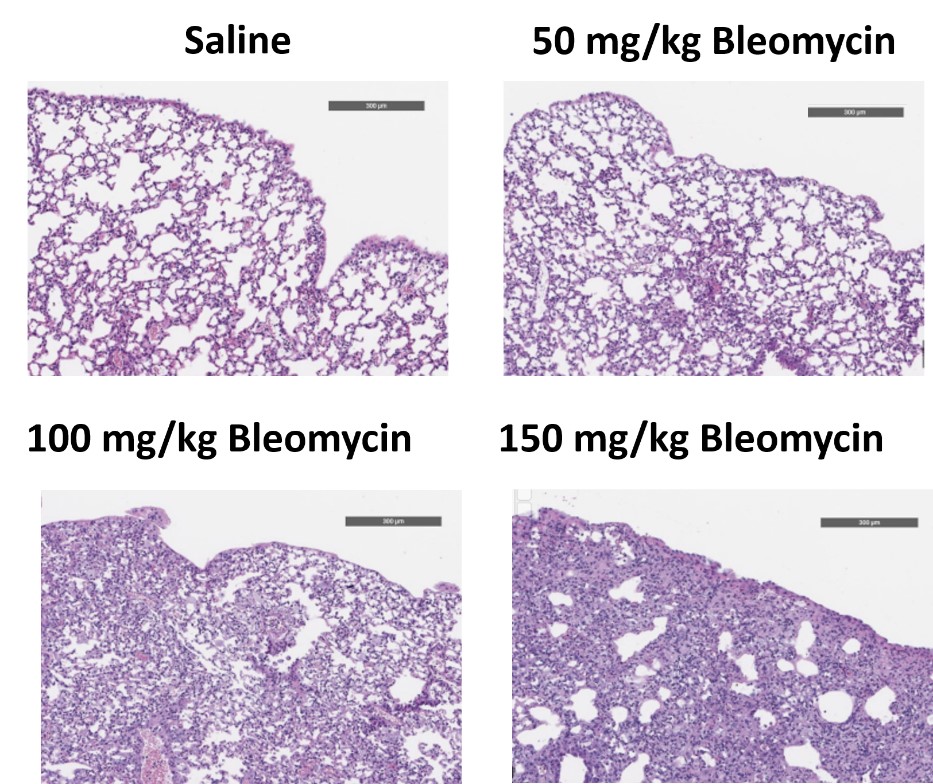
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and Day 28 lungs are processed for histopathology. H&E-stained lungs from animals administered saline or 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin.
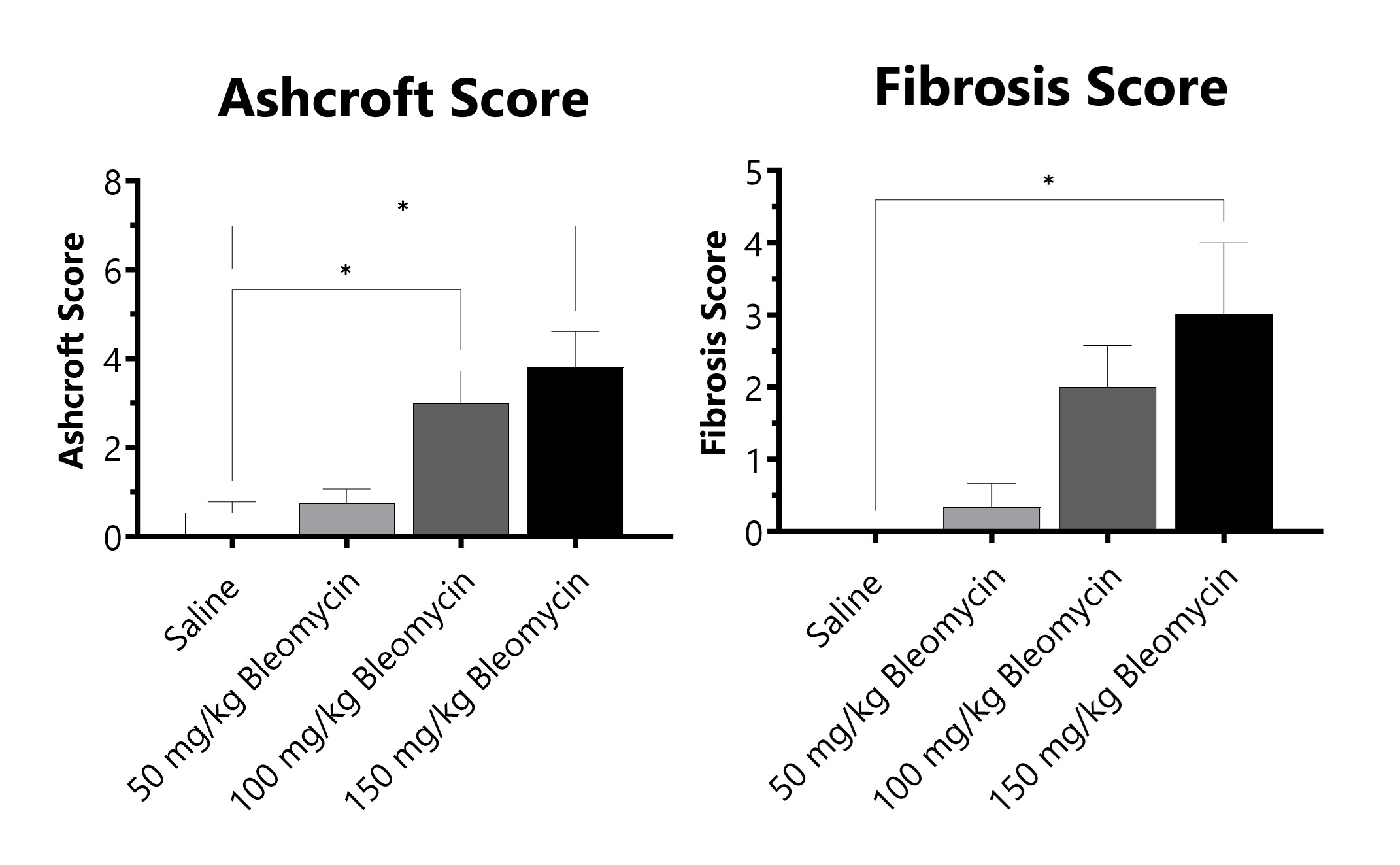
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump. Pathology assessment of Day 28 lungs from SC Osmotic Pump-Bleomycin administered animals. Ashcroft score is determined in H&E-stained tissue using a modified Ashcroft Scale graded from 0-8. Fibrosis score is determined in Masson’s Trichrome-stained tissue using a 0-5 semi-quantitative scoring scale of area staining positive for collagen. (*p<0.05 compared to the saline-control).
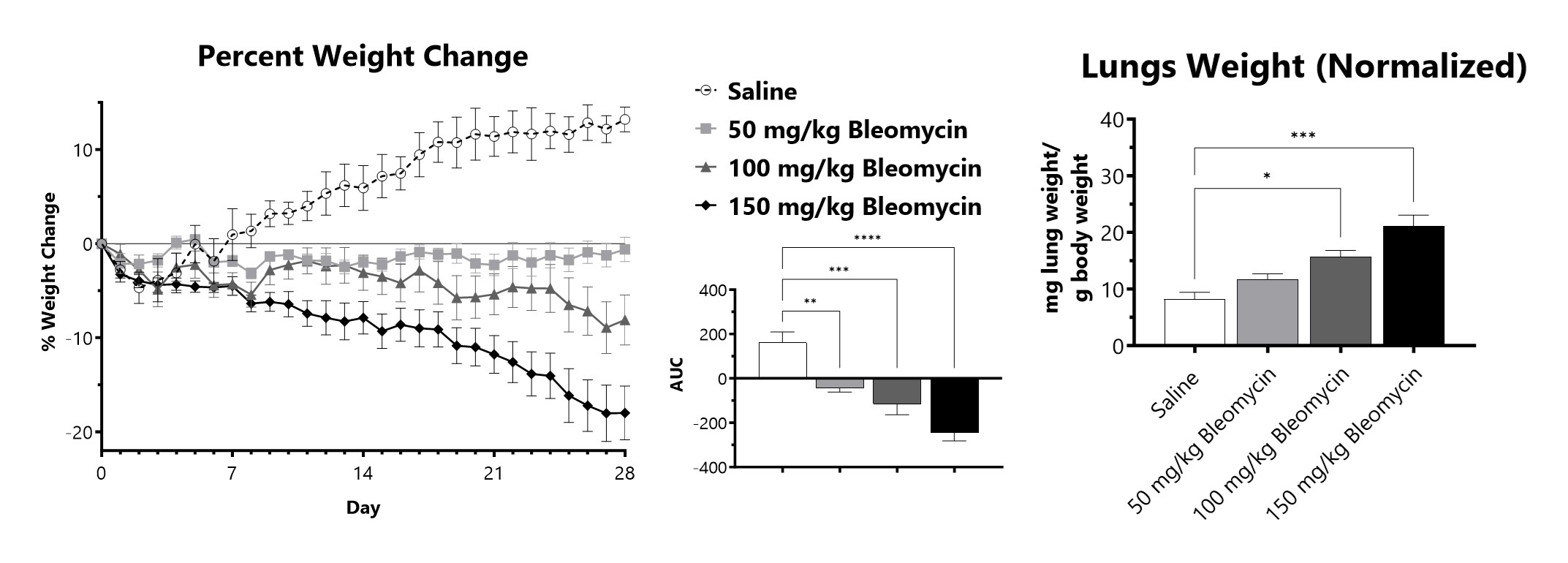
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump. Animals are weighed daily, and body weight compared to Day 0 is calculated. The AUC is calculated to compare treatment arms and is shown in the inset. Wet lung weight normalized to Day 28 body weight of Day 28 lungs from SC Osmotic Pump-Bleomycin administered animals. (*p<0.05; ***p<0.001 compared to the saline-control).
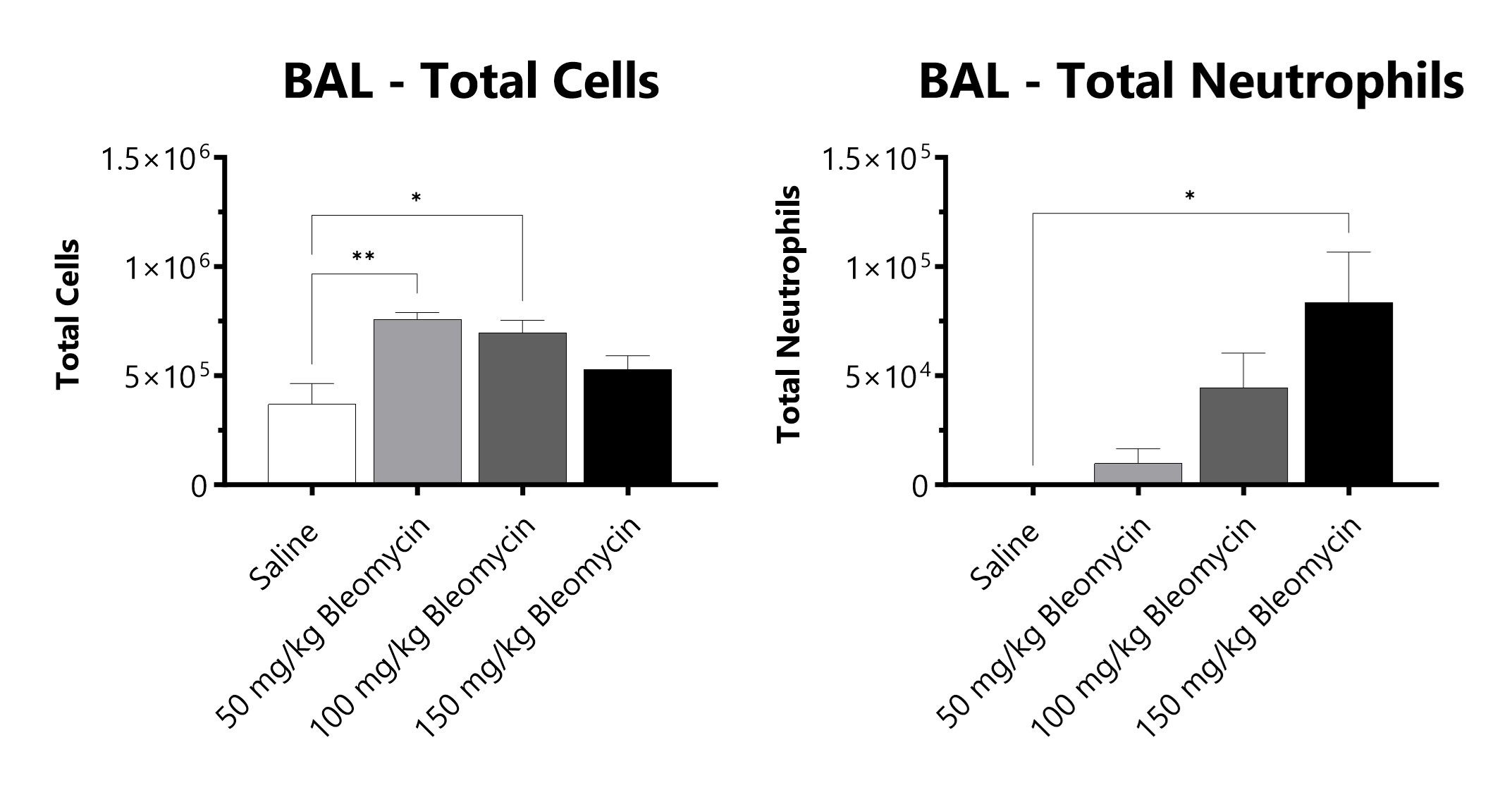
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and BAL fluid is assessed at 28 days for total cells and total neutrophils following administration of either 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin. (*p<0.05; **p<0.01 compared to the saline-control).
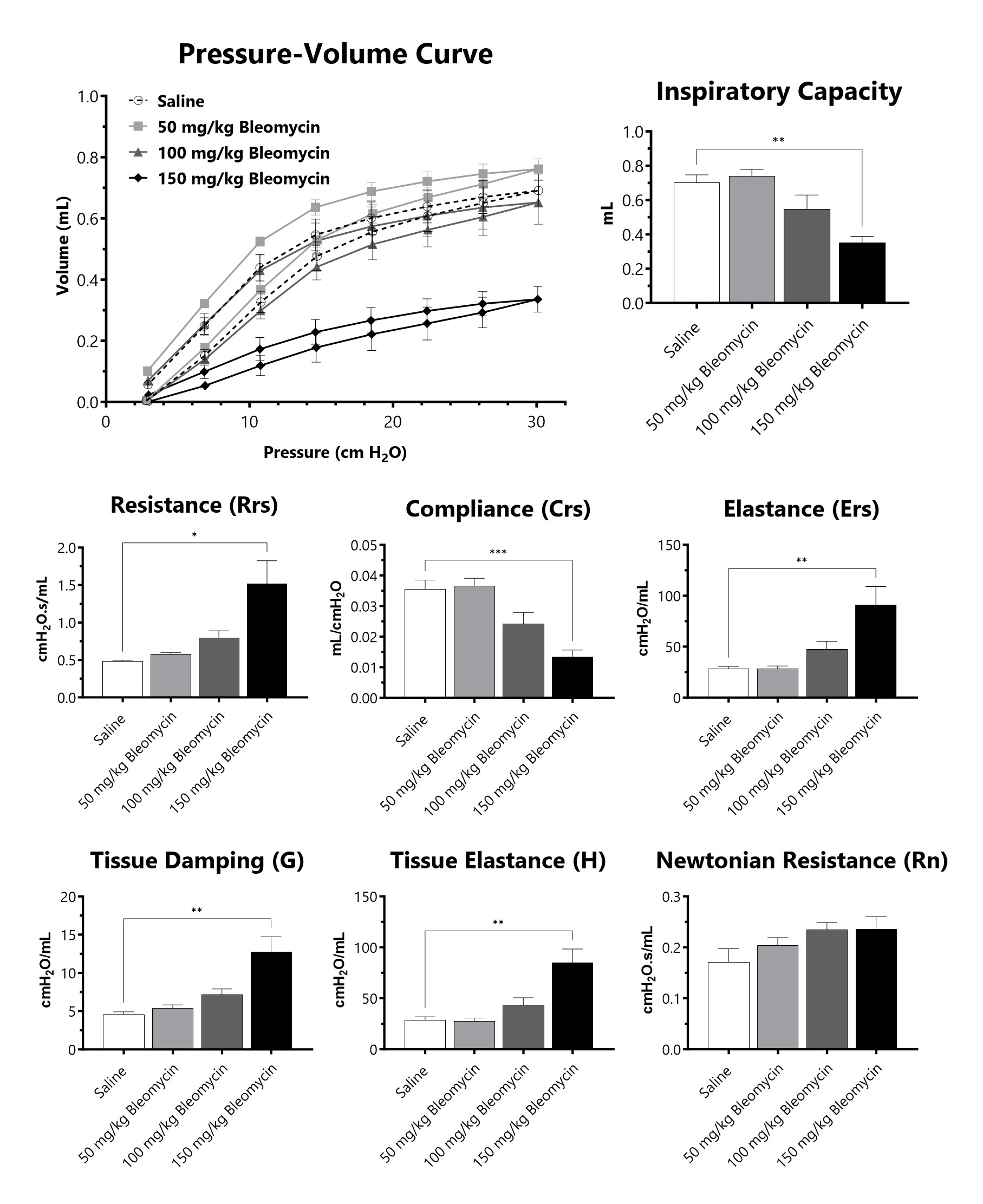
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and lung mechanics are assessed at 28 days following administration of either 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin. (*p<0.05; **p<0.01; ***p<0.001 compared to the saline-control).
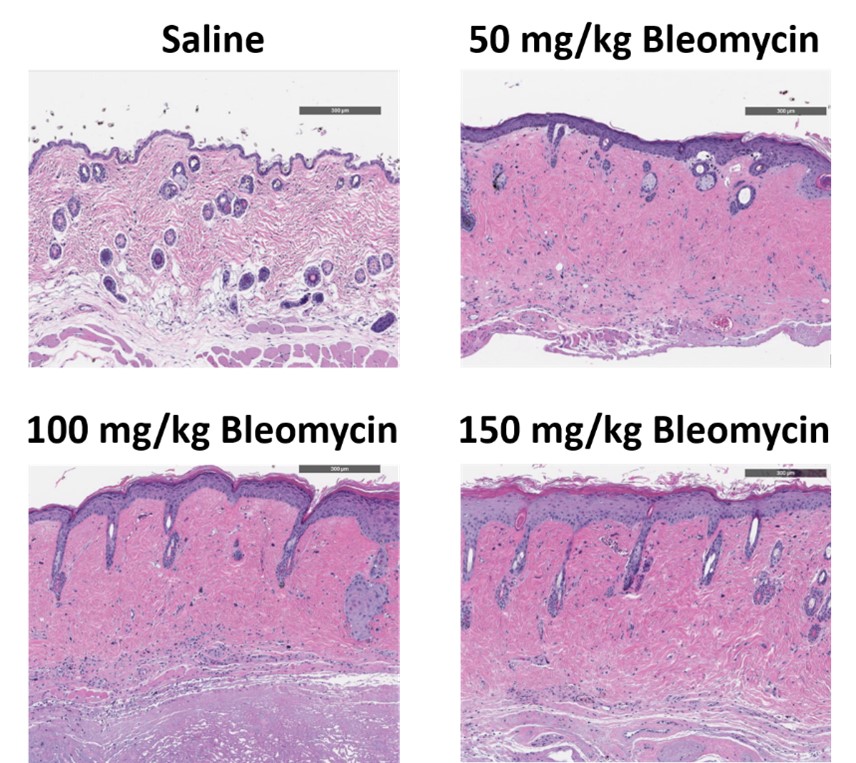
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump and Day 28 skin samples are processed for histopathology. H&E-stained skin from animals administered saline or 50 mg/kg, 100 mg/kg, or 150 mg/kg Bleomycin.
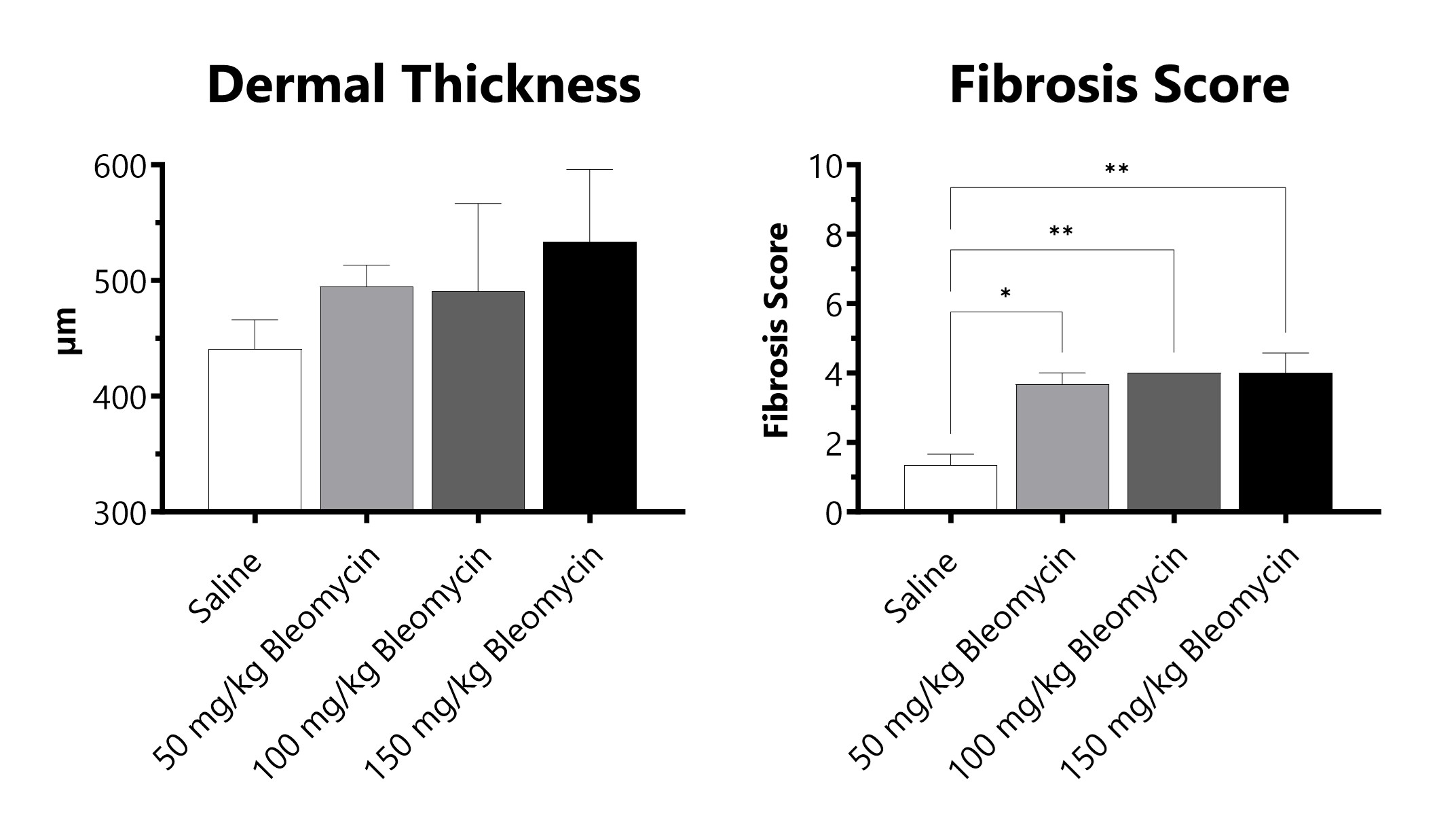
C57Bl/6 mice are administered Bleomycin for 28 days through a SC implanted osmotic pump. Pathology assessment of Day 28 skin from SC Osmotic Pump-Bleomycin administered animals. Dermal thickness is measured, and fibrosis score is determined in Masson’s Trichrome-stained tissue using a 0-5 semi-quantitative scoring scale of area staining positive for collagen. (*p<0.05; **p<0.01 compared to the saline-control).

Close